Quercetin
From Wikipedia, the free encyclopedia
| Quercetin | |
|---|---|
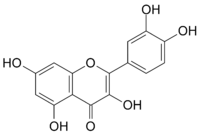 |
|
| IUPAC name |
|
| Identifiers | |
| CAS number | 117-39-5 |
| PubChem | |
| SMILES |
|
| Properties | |
| Molecular formula | C15H10O7 |
| Molar mass | 302.236 g/mol |
| Density | 1.799 g/cm3 |
| Melting point |
316 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Quercetin is a plant-derived flavonoid, specifically a flavonol, used as a nutritional supplement.
The American Cancer Society says that quercetin "has been promoted as being effective against a wide variety of diseases, including cancer. While some early lab results appear promising, as of yet there is no reliable clinical evidence that quercetin can prevent or treat cancer in humans." In the amounts consumed in a healthy diet, quercetin "is unlikely to cause any major problems."[1]
High dietary intake of fruits and vegetables is associated with reduction in cancer, and scientists suspect quercetin may be partly responsible. Research shows that quercetin influences cellular mechanisms in vitro and in animal studies, and there is limited evidence from human population studies that quercetin may reduce the risk of lung cancer.[2][3]
Some researchers believe quercetin should not be used by healthy people (for prevention) until it can be shown that quercetin doesn't itself cause cancer. In laboratory studies of cells (in vitro), quercetin produces changes that are also produced by compounds that cause cancer (carcinogens), but these studies don't report increased cancer in animals or humans.[4][5][6] The U.S. Food and Drug Administration has not approved any health claims for quercetin.[7] There is current early-stage clinical research on quercetin addressing safety and efficacy against sarcoidosis, asthma and glucose absorption in obesity and diabetes (February 2009).[8]
Quercetin is the aglycone form of a number of other flavonoid glycosides, such as rutin and quercitrin, found in citrus fruit, buckwheat and onions. Quercetin forms the glycosides quercitrin and rutin together with rhamnose and rutinose, respectively. Quercetin is classified as IARC group 3 (no evidence of carcinogenicity in humans).
Contents |
[edit] Occurrence
Quercetin is a naturally-occurring polar auxin transport inhibitor.[citation needed]
Foods rich in quercetin include capers (1800mg/kg)[9], lovage (1700mg/kg), apples (440mg/kg), tea (Camellia sinensis), onion, especially red onion (higher concentrations of quercetin occur in the outermost rings[10]), red grapes, citrus fruit, tomato, broccoli and other leafy green vegetables, and a number of berries including cherry, raspberry, bog whortleberry (158 mg/kg, fresh weight), lingonberry (cultivated 74mg/kg, wild 146 mg/kg), cranberry (cultivated 83 mg/kg, wild 121 mg/kg), chokeberry (89 mg/kg), sweet rowan (85 mg/kg), rowanberry (63 mg/kg), sea buckthorn berry (62 mg/kg), crowberry (cultivated 53mg/kg, wild 56 mg/kg),[11] and the fruit of the prickly pear cactus. A recent study found that organically grown tomatoes had 79% more quercetin than "conventionally grown".[12]
A study[13] by the University of Queensland, Australia, has also indicated the presence of quercetin in varieties of honey, including honey derived from eucalyptus and tea tree flowers.[14]
[edit] Possible medicinal properties
From in vitro studies, quercetin has demonstrated significant anti-inflammatory activity by inhibiting both manufacture and release of histamine and other allergic/inflammatory mediators.[citation needed] In addition, it exerts potent antioxidant activity and vitamin C-sparing action[citation needed].
Quercetin also shows anti-tumor properties in vitro. When treated with a combination of quercetin and ultrasound at 20 kHz for 1 minute duration, skin and prostate cancers show a 90% mortality within 48 hours with no visible mortality of normal cells.[15] Note that ultrasound also promotes topical absorption by up to 1,000 times making the use of topical quercetin and ultrasound wands an interesting proposition.
Recent studies have supported that quercetin may help men with chronic prostatitis, and both men and women with interstitial cystitis, possibly because of its action as a mast cell inhibitor.[16]
Quercetin may have positive effects in combating or helping to prevent cancer, prostatitis, heart disease, cataracts, allergies/inflammations, and respiratory diseases such as bronchitis and asthma[citation needed]. It also has been claimed to have antidepressant properties, however any claim of quercetin action against neurological diseases should be treated with skepticism due to the fact that quercetin is a neurotoxin in vitro.[17]
It has also been claimed that quercetin reduces blood pressure in hypertensive subjects.[18]
It also may be found in dietary supplements.
An 8-year study found that three flavonols — kaempferol, quercetin, and myricetin — were associated with a reduced risk of pancreatic cancer of 23 percent.[19]
A laboratory study found that a dose of 12.5 to 25 mg/kg increased endurance in mice by causing more mitochondria to grow.[20]
An in-vitro study on fat cells suggested that quercetin and resveratrol may have a synergistic anti-obesity effect.[21]
Despite these indications of possible health benefits, quercetin has neither been confirmed as a specific therapeutic for any condition nor has it been approved by any regulatory agency. In fact, bioavailability studies show that ingested quercetin is extensively metabolized into non-active phenolic acids, with more than 96% of the ingested amount excreted within 72 hours, indicating actual physiological roles, if they exist, involve quercetin in only minute amounts.[22]
[edit] Drug interactions
Quercetin is contraindicated with some antibiotics; it may interact with fluoroquinolones (a type of medicinal antibiotic), as quercetin competitively binds to bacterial DNA gyrase. Whether this inhibits or enhances the effect of fluoroquinolones is not entirely clear.[23]
Quercetin is also a potent inhibitor of CYP3A4[24] and CYP2C9[25], which are enzymes that break down most drugs in the body. As such, quercetin would be expected to increase serum levels, and therefore effects, of drugs metabolized by this enzyme.
In cattle there is a synergystic interaction between Bovine Papillomavirus-2 infection and exposure to Quercetin, promoting bladder neoplasia, clinically presenting as enzootic haematuria. A similar effect is seen on exposure to the bracken fern Pteridium aqualinum, and the chemical Ptaquiloside found within it.
[edit] See also
[edit] References
- ^ American Cancer Society, Quercetin
- ^ Neuhouser ML. Dietary flavonoids and cancer risk: evidence from human population studies. Nutr Cancer. 2004;50(1):1-7. PMID 15572291
- ^ Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008 Oct 8;269(2):315-25.[1]
- ^ Verschoyle RD, Steward WP, Gescher AJ. Putative cancer chemopreventive agents of dietary origin-how safe are they? Nutr Cancer. 2007;59(2):152-62.[2]
- ^ Rietjens IM, Boersma MG, van der Woude H, Jeurissen SM, Schutte ME, Alink GM. Flavonoids and alkenylbenzenes: mechanisms of mutagenic action and carcinogenic risk. Mutat Res. 2005 Jul 1;574(1-2):124-38.[3]
- ^ van der Woude H et al. Formation of transient covalent protein and DNA adducts by quercetin in cells with and without oxidative enzyme activity. Chem Res Toxicol. 2005 Dec;18(12):1907-16.[4]
- ^ US FDA, Center for Food Safety and Nutrition, Qualified Health Claims Subject to Enforcement Discretion, April 2007[5]
- ^ Clinicaltrials.gov, National Institutes of Health, [6]
- ^ USDA Database for the Flavonoid Content of Selected Foods
- ^ Crystal Smith, Kevin A. Lombard, Ellen B. Peffley, Weixin Liu (2003). "Genetic Analysis of Quercetin in Onion (Allium cepa L.) Lady Raider" ([dead link]). The Texas Journal of Agriculture and Natural Resource (Agriculture Consortium of Texas) 16: 24–28. http://www.tarleton.edu/~txjanr/2003issue/article3.pdf.
- ^ Sari H. Häkkinen et al (1999). "Content of the Flavonols Quercetin, Myricetin, and Kaempferol in 25 Edible Berries". Journal of Agricultural and Food Chemistry 47 (6): 2274–2279. doi:. PMID 10794622.
- ^ A. E. Mitchell, Y. J. Hong, E. Koh, D. M. Barrett, D. E. Bryant, R. F. Denison and S. Kaffka (2007). "Ten-Year Comparison of the Influence of Organic and Conventional Crop Management Practices on the Content of Flavonoids in Tomatoes". Journal of Agricultural and Food Chemistry 55 (15): 6154–6159. doi:.
- ^ Honey Research Unit
- ^ honey fingerprinting
- ^ "Induction of cancer-specific cytotoxicity towards human prostate and skin cells using quercetin and ultrasound" (abstract). British Journal of Cancer 92 (3): 499–502. 2005. doi:. http://www.nature.com/bjc/journal/v92/n3/abs/6602364a.html. Retrieved on 2007-05-19.
- ^ Shoskes, DA et al (1999). "Quercetin in men with category III chronic prostatitis: a preliminary prospective, double-blind, placebo-controlled trial". Urology. 54 (6): 960–3. doi:. PMID 10604689.
- ^ "Paradoxical co-existence of protective and toxic effects of quercetin in the same in vitro neurodegeneration model". European Journal of Pharmaceutical Sciences 34 (1): S33. 2008. doi:.
- ^ "Quercetin Reduces Blood Pressure in Hypertensive Subjects". Journal of Nutrition 137: 2405-2411. 2007.
- ^ Ute Nöthlings, Suzanne P. Murphy, Lynne R. Wilkens, Brian E. Henderson3 and Laurence N. Kolonel (2007). "Flavonols and Pancreatic Cancer Risk". American Journal of Epidemiology 166 (8): 924–931. doi:. PMID 17690219.
- ^ Davis JM, Murphy EA, Carmichael MD, Davis B. (2009). "Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance.". Am J Physiol Regul Integr Comp Physiol.. PMID 19211721.
- ^ Yang JY, Della-Fera MA, Rayalam S, Ambati S, Hartzell DL, Park HJ, Baile CA (2008). "Enhanced inhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin". Life Sci. 82 (19-20): 1032-9. PMID 18433793.
- ^ Mullen W et al. (Dec 2008). "Bioavailability of [2-(14)C]quercetin-4'-glucoside in rats.". J Agric Food Chem. 2456 (24): 12127-37. PMID 19053221.
- ^ Hilliard JJ, Krause HM, Bernstein JI, Fernandez JA, Nguyen V, Ohemeng KA, Barrett JF. 'A comparison of active site binding of 4-quinolones and novel flavone gyrase inhibitors to DNA gyrase. Adv Exp Med Biol. 1995;390:59-69. PMID 8718602.
- ^ Su-Lan Hsiu; Yu-Chi Hou; Yao-Horng Wang; Chih-Wan Tsao; Sheng-Fang Sue; and Pei-Dawn L. Chao (6 December 2002). "Quercetin significantly decreased cyclosporin oral bioavailability in pigs and rats". Life Sciences 72 (3): 227–235. doi:.
- ^ Si Dayong, Wang Y, Zhou Y-H, Guo Y, Wang J, Zhou H, Li Z-S, Fawcett JP (March 2009). "Mechanism of CYP2C9 inhibition by flavones and flavonols". Drug Metabolism and Disposition 37: 629-634.. doi:. http://p4502c.googlepages.com/dmd2.pdf.
[edit] External links
- UMM Quercetin Info Page (University of Maryland Medical Center Website)
- Eyes right for a cup of tea UK Institute of Food Research article on how quercetin can help prevent cataracts.
- Plant foods for health protection article by the Institute of Food Research (Norwich, United Kingdom)
- Scanning Electron Micrograph image of quercetin crystals derived from onion
- Possible Interactions with: Quercetin Quercetin might enhance the effects of two chemotherapy medications
- Quercetin Info from the Cedars-Sinai Medical Center, Los Angeles.
- "Genetic Analysis of Quercetin in Onion (Allium cepa L.)" article from The Texas Journal of Agriculture and Natural Resource 16:24-28 (2003).
- "Ten-Year Comparison of the Influence of Organic and Conventional Crop Management Practices on the Content of Flavonoids in Tomatoes" article at American Chemical Society website (originally published 23 June 2007).
- "An apple a day could help protect against brain-cell damage" article at Cornell University website (originally published 2 December 2004).
|
|||||||||||||||||||||||||||||

