Capsaicin
From Wikipedia, the free encyclopedia
| Capsaicin | |
|---|---|
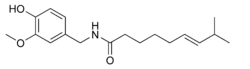 |
|
 |
|
| IUPAC name |
|
| Other names | (E)-N-(4-Hydroxy-3-methoxybenzyl) -8-methylnon-6-enamide, trans-8-Methyl-N-vanillylnon -6-enamide, (E)-Capsaicin, CPS, C |
| Identifiers | |
| CAS number | 404-86-4 |
| PubChem | |
| EC number | |
| ATC code | M02 |
| SMILES |
|
| ChemSpider ID | |
| Properties | |
| Molecular formula | C18H27NO3 |
| Molar mass | 305.41 g/mol |
| Melting point |
62–65 °C |
| Boiling point |
210–220 °C @ 0.01 Torr |
| Hazards | |
| MSDS | [1] |
| Main hazards | Toxic (T) |
| NFPA 704 | |
| R-phrases | R24/25 |
| S-phrases | S26, S36/37/39, S45 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
| Heat: Above Peak (SR: 15,000,000-16,000,000) |
Capsaicin (pronounced /ˌkæpˈseˌɪ.sɪn/) (8-methyl-N-vanillyl-6-nonenamide, (CH3)2CHCH=CH(CH2)4CONHCH2C6H3-4-(OH)-3-(OCH3)) is the active component of chili peppers, which are plants belonging to the genus Capsicum. It is an irritant for mammals, including humans, and produces a sensation of burning in any tissue with which it comes into contact. Capsaicin and several related compounds are called capsaicinoids and are produced as a secondary metabolite by chili peppers, probably as deterrents against certain herbivores and fungi.[1] Pure capsaicin is a hydrophobic, colorless, odorless, crystalline to waxy compound.
Contents |
[edit] History
The molecule was first isolated in 1816 in crystalline form by P. A. Bucholz and again 30 years later by L.T. Thresh, who gave it the name "capsaicin".[2] In 1878, the Hungarian doctor Endre Hőgyes (calling it capsicol) isolated it and proved that it not only caused the burning feeling when in contact with mucous membranes but also increased secretion of gastric juice. The structure of capsaicin was partly elucidated by E. K. Nelson in 1919.[3] Capsaicin was first synthesized in 1930 by E. Spath and F. S. Darling. In 1961, similar substances were isolated from chili peppers by the Japanese chemists S. Kosuge and Y. Inagaki, who named them capsaicinoids.[4][5]
[edit] Capsaicinoids
Capsaicin is the main capsaicinoid in chili peppers, followed by dihydrocapsaicin. These two compounds are also about twice as potent to the taste and nerves as the minor capsaicinoids nordihydrocapsaicin, homodihydrocapsaicin, and homocapsaicin. Dilute solutions of pure capsaicinoids produced different types of pungency; however, these differences were not noted using more concentrated solutions.
Capsaicin is believed to be synthesized in the interlocular septa of chili peppers by addition of a branched-chain fatty acid to vanillylamine. Biosynthesis depends on the gene AT3, which resides at the pun1 locus, and which encodes a putative acyltransferase.[6]
Besides the six natural capsaicinoids, one synthetic member of the capsaicinoid family exists. Vanillylamide of n-nonanoic acid (VNA) is used as a reference substance for determining the relative pungency of capsaicinoids.
| Capsaicinoid name | Abbrev. | Typical relative amount |
Scoville heat units |
Chemical structure |
|---|---|---|---|---|
| Capsaicin | C | 69% | 16,000,000 | 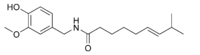 |
| Dihydrocapsaicin | DHC | 22% | 15,000,000 |  |
| Nordihydrocapsaicin | NDHC | 7% | 9,100,000 |  |
| Homodihydrocapsaicin | HDHC | 1% | 8,600,000 |  |
| Homocapsaicin | HC | 1% | 8,600,000 |  |
| Nonivamide | PAVA |  |
[edit] Natural function
Capsaicin is present in large quantities in the placental tissue (which holds the seeds), the internal membranes and, to a lesser extent, the other fleshy parts of the fruits of plants in the genus Capsicum. Contrary to popular belief, the seeds themselves do not produce any capsaicin, although the highest concentration of capsaicin can be found in the white pith around the seeds.[7]
The seeds of Capsicum plants are predominantly dispersed by birds. Birds do not have the receptor to which capsaicin binds, so it does not function as an irritant for them. Chili pepper seeds consumed by birds pass through the digestive tract and can germinate later, but mammals have molars, which destroy seeds and prevent them from germinating. Thus, natural selection may have lead to increasing capsaicin production because it makes the plant less likely to be eaten by animals that do not help it reproduce. However, there is evidence that capsaicin first evolved as an anti-fungal agent.[1]
In 2006 it was discovered that tarantula venom activates the same pathway of pain as is activated by capsaicin, the first demonstrated case of such a shared pathway in both plant and animal anti-mammal defense.[8]
[edit] Uses
[edit] Food
Because of the burning sensation caused by capsaicin when it comes in contact with mucous membranes, it is commonly used in food products to give them added spice or "heat" (pungency). In high concentrations capsaicin will also cause a burning effect on other sensitive areas of skin. The degree of heat found within a food is often measured on the Scoville scale.
Cooling and mechanical stimulation are the only proven methods to relieve the pain. The burning sensation will slowly fade away if no actions are taken.
It is common for people to experience pleasurable and even euphoriant effects from eating capsaicin-flavored foods. Folklore among self-described "pepperheads" attributes this to pain-stimulated release of endorphins, a different mechanism from the local receptor overload that makes capsaicin effective as a topical analgesic. In support of this theory, there is some evidence that the effect can be blocked by naloxone and other compounds that compete for receptor sites with endorphins and opiates.[9]
[edit] Medical
Capsaicin is currently used in topical ointments to relieve the pain of peripheral neuropathy such as post-herpetic neuralgia caused by shingles. It may be used in concentrations of between 0.025% and 0.075%. It may be used as a cream for the temporary relief of minor aches and pains of muscles and joints associated with arthritis, simple backache, strains and sprains. The treatment typically involves the application of a topical anesthetic until the area is numb. Then the capsaicin is applied by a therapist wearing rubber gloves and a face mask. The capsaicin remains on the skin until the patient starts to feel the "heat", at which point it is promptly removed. Capsaicin is also available in large bandages that can be applied to the back.
Recently, capsaicin is being tested for the prevention of pain post surgery. David Julius, a physiology professor at the University of California, San Francisco, recently discovered that capsaicin selectively binds to a protein known as TRPV1 that resides on the membranes of pain and heat sensing neurons. TRPV1 a heat activated calcium channel, with a threshold to open between 37 and 45 Celsius degrees (37 degrees is normal body temperature). When capsaicin binds to TRPV1, it causes the channel to lower its opening threshold, thereby opening it at temperatures less than the body's temperature, which is why capsaicin is linked to the sensation of heat. Prolonged activation of these neurons by capsaicin depletes presynaptic substance P, one of the body's neurotransmitters for pain and heat. Neurons that do not contain TRPV1 are unaffected.[10] This causes extended numbness following surgery, and the patient does not feel pain as the capsaicin is applied under anesthesia.
The result appears to be that the chemical mimics a burning sensation, the nerves are overwhelmed by the influx, and are unable to report pain for an extended period of time. With chronic exposure to capsaicin, neurons are depleted of neurotransmitters and it leads to reduction in sensation of pain and blockade of neurogenic inflammation. If capsaicin is removed, the neurons recover.[citation needed]
Capsaicin is being explored as a possible prophylaxis for Type 1 diabetes by researchers in Toronto, Canada; capsaicin was injected subcutaneously affecting pancreatic sensory nerves of mice with Type 1 diabetes because of a suspected link between the nerves and diabetes.[11]
The American Association for Cancer Research reports studies suggesting capsaicin is able to kill prostate cancer cells by causing them to undergo apoptosis.[12][13] The studies were performed on tumors formed by human prostate cancer cell cultures grown in mouse models, and showed tumors treated with capsaicin were about one-fifth the size of the untreated tumors. There have been several clinical studies conducted in Japan and China that showed natural capsaicin directly inhibits the growth of leukemic cells.[14]
Another study carried out at the University of Nottingham suggests capsaicin is able to trigger apoptosis in human lung cancer cells as well.[15]
Capsaicin is also the key ingredient in the experimental drug Adlea, which is in Phase 2 trials as a long-acting analgesic to treat post-surgical and osteoarthritis pain for weeks to months after a single injection to the site of pain.[16]
[edit] Proposed drug abuse deterrent
Clifford Woolf, the Richard J. Kitz Professor of Anesthesia Research at Harvard Medical School, has suggested using capsaicin to deter abuse of certain extended-release drugs such as OxyContin and Ritalin.[17] When taken as prescribed, opioid prescription drugs such as OxyContin or stimulant drugs such as Adderall XR release their active chemical over time, but when crushed and snorted, taken as a suppository, chewed, or injected, the larger than normal dosage is absorbed all at once and a much stronger effect is produced that can be highly habit forming and dangerous due to the higher risk of overdose. Woolf has argued that adding capsaicin into the capsules would be a safe way to deter abuse. A person taking the capsule in the prescribed way (i.e., swallowing it whole) would suffer no ill effects from the additive. However, a person crushing it would expose the irritant. Anyone then chewing it, snorting it, or injecting it would be exposed to the full power of the chemical. "Imagine snorting an extract of 50 jalapeño peppers and you get the idea," Woolf said in an interview with the Harvard University Gazette. As of 2006, Woolf's proposal is still in the preliminary stages of development and the additive has not yet entered the production stage.
[edit] Non-lethal force
Capsaicin is also the active ingredient in riot control and personal defense pepper spray chemical agents. When the spray comes in contact with skin, especially eyes or mucous membranes, it is very painful, and breathing small particles of it as it disperses can cause breathing difficulty, which serves to discourage assailants. Refer to the Scoville scale for a comparison of pepper spray to other sources of capsaicin.
In large quantities, capsaicin can cause death.[18] Symptoms of overdose include difficulty breathing, blue skin, and convulsions. The large amount needed to kill an adult human and the low concentration of capsaicin in chilies make the risk of accidental poisoning by chili consumption negligible.
[edit] Pest deterrent
Capsaicin is also used to deter mammalian pests. A common example is the use of ground-up or crushed dried chili pods in birdseed to deter squirrels, since birds are unaffected by capsaicin. Another example is the use of chili peppers by the Elephant Pepper Development Trust to improve crop security for rural communities in Africa.
[edit] Equestrian sports
Capsaicin is a banned substance in equestrian sports because of its hypersensitizing and pain relieving properties. At the show jumping events of the 2008 Summer Olympics, four horses tested positive for the substance, resulting in disqualification.[19]
[edit] Mechanism of action
The burning and painful sensations associated with capsaicin result from its chemical interaction with sensory neurons. Capsaicin, as a member of the vanilloid family, binds to a receptor called the vanilloid receptor subtype 1 (VR1).[20] First cloned in 1997, VR1 is an ion channel-type receptor. VR1, which can also be stimulated with heat and physical abrasion, permits cations to pass through the cell membrane and into the cell when activated. The resulting depolarization of the neuron stimulates it to signal the brain. By binding to the VR1 receptor, the capsaicin molecule produces the same sensation that excessive heat or abrasive damage would cause, explaining why the spiciness of capsaicin is described as a burning sensation.
The VR1 ion channel has subsequently been shown to be a member of the superfamily of TRP ion channels, and as such is now referred to as TRPV1. There are a number of different TRP ion channels that have been shown to be sensitive to different ranges of temperature and probably are responsible for our range of temperature sensation. Thus, capsaicin does not actually cause a chemical burn, or indeed any damage to tissue at all; it causes only the sensation of one.
[edit] Toxicity
[edit] Acute health effects
Capsaicin is a highly irritant material requiring proper protective goggles, respirators, and proper hazardous material handling procedures. It is hazardous in cases of skin contact (irritant, sensitizer), of eye contact (irritant), of ingestion, of inhalation (lung irritant, lung sensitizer). Severe over-exposure to pure capsaicin can result in death; the lethal dose (LD50 in mice) is 47.2 mg/kg.[18] Numerous other adverse health effects can occur in mammals.[21]
Painful exposures to capsaicin-containing peppers are among the most common plant-related exposures presented to poison centers.[22] They cause burning or stinging pain to the skin, and if ingested in large amounts by adults or small amounts by children, can produce nausea, vomiting, abdominal pain and burning diarrhea.[22] Eye exposure produces intense tearing, pain, conjunctivitis and blepharospasm.[22]
[edit] Treatment after exposure
The primary treatment is removal from exposure. Contaminated clothing should be removed and placed in airtight bags to prevent secondary exposure. Capsaicin could be washed off the skin using soap, shampoo, or other detergents, or rubbed off with oily compounds such as vegetable oil, paraffin oil, petroleum jelly (Vaseline), creams, or polyethylene glycol. Plain water, as well as home remedies such as vinegar, bleach, sodium metabisulfite, or topical antacid suspensions are ineffective in removing capsaicin.
Burning and pain symptoms can be effectively relieved by cooling, e.g., from ice, cold water, cold bottles, cold surfaces, or a flow of air from wind or a fan. In severe cases, eye burn might be treated symptomatically with topical ophthalmic anesthetics; mucous membrane burn with lidocaine gel. Capsaicin-induced asthma might be treated with nebulized bronchodilators or oral antihistamines or corticosteroids.[22]
[edit] Effects of dietary consumption
Ingestion of spicy food or ground jalapeño peppers does not cause mucosal erosions or other abnormalities.[23] Some mucosal microbleeding has been found after eating red and black peppers, but there was no significant difference between aspirin (used as a control) and peppers.[24] An association was found between chronic consumption of capsaicin-rich foods and stomach cancer in a group of Mexican patients.[25] By use of county population and mortality data, significantly higher rates for stomach and liver cancer were found in counties inhabited by groups with high consumption of capsaicin-rich foods than in matched control counties.[26]
[edit] References
[edit] Footnotes
- ^ a b What Made Chili Peppers So Spicy? Talk of the Nation, 15 Aug 2008.
- ^ J King, H Wickes Felter, J Uri Lloyd (1905) A King's American Dispensatory. Eclectic Medical Publications (ISBN 1888483024)
- ^ E. K. Nelson. The constitution of capsaicin, the pungent principle of capsicum. J. Am. Chem. Soc. 1919, 41, 1115–1121. doi:10.1021/ja02228a011
- ^ S Kosuge, Y Inagaki, H Okumura (1961). Studies on the pungent principles of red pepper. Part VIII. On the chemical constitutions of the pungent principles. Nippon Nogei Kagaku Kaishi (J. Agric. Chem. Soc.), 35, 923–927; (en) Chem. Abstr. 1964, 60, 9827g.
- ^ (ja) S Kosuge, Y Inagaki (1962) Studies on the pungent principles of red pepper. Part XI. Determination and contents of the two pungent principles. Nippon Nogei Kagaku Kaishi (J. Agric. Chem. Soc.), 36, pp. 251
- ^ Stewart C, Kang BC, Liu K, et al (June 2005). "The Pun1 gene for pungency in pepper encodes a putative acyltransferase". Plant J. 42 (5): 675–88. doi:. PMID 15918882.
- ^ New Mexico State University - College of Agriculture and Home Economics (2005). [http://spectre.nmsu.edu/dept/academic.html?i=1274&s=sub "Chile Information - Frequently Asked Questions"]. http://spectre.nmsu.edu/dept/academic.html?i=1274&s=sub. Retrieved on May 17.
- ^ Siemens J, Zhou S, Piskorowski R, et al (November 2006). "Spider toxins activate the capsaicin receptor to produce inflammatory pain". Nature 444 (7116): 208–12. doi:. PMID 17093448.
- ^ http://www.liebertonline.com/doi/abs/10.1089/10755530260128032?cookieSet=1&journalCode=acm
- ^ Chili Pepper Cocktail Blunts Pain: Scientific American
- ^ Razavi R, Chan Y, Afifiyan FN, et al (December 2006). "TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes". Cell 127 (6): 1123–35. doi:. PMID 17174891.
- ^ Mori, A; Lehmann S, O'Kelly J et al. (March 2006). "Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells". Cancer Research (American Association for Cancer Research) 66 (6): 3222–3229. doi:. PMID 16540674. http://cancerres.aacrjournals.org/cgi/content/full/66/6/3222. Retrieved on 2008-07-22.
- ^ American Association for Cancer Research (2006). "Pepper component hot enough to trigger suicide in prostate cancer cells". http://www.eurekalert.org/pub_releases/2006-03/aafc-pch031306.php. Retrieved on January 27.
- ^ Ito, K; Nakazato T, Yamato K et al. (February 2004). "Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species". Cancer Research (American Association for Cancer Research) 64 (3): 1071–1078. doi:. PMID 14871840. http://cancerres.aacrjournals.org/cgi/content/full/64/3/1071. Retrieved on 2008-07-22.
- ^ BBC News (2007). "How spicy foods can kill cancers". http://news.bbc.co.uk/2/hi/health/6244715.stm. Retrieved on January 09.
- ^ "Doctors Test Hot Sauce For Pain Relief". http://www.mail.com/Article.aspx?articlepath=APNews%5CTop%20Headlines%5C20071030%5CHealthBeat_Peppers___Pain_20071030.xml&cat=topheadlines&subcat=&pageid=1. Retrieved on 2007-10-30.
- ^ Cromie WJ (2006) "Using chilli peppers to burn drug abusers" Harvard University Gazette accessed 24 January 2006
- ^ a b "Capsaicin Material Safety Data Sheet" (pdf). sciencelab.com. 2007. http://www.sciencelab.com/xMSDS-Capsaicin_Natural-9923296. Retrieved on 2007-07-13.
- ^ http://news.bbc.co.uk/sport1/hi/olympics/equestrian/7574220.stm
- ^ Story GM, Crus-Orengo L (July–August 2007). "Feel the burn". American Scientist 95 (4): 326–333. doi:.
- ^ Johnson, Wilbur (2007). "Final report on the safety assessment of capsicum annuum extract, capsicum annuum fruit extract, capsicum annuum resin, capsicum annuum fruit powder, capsicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin". Int. J. Toxicol. 26 Suppl 1: 3–106. doi:. PMID 17365137.
- ^ a b c d Goldfrank, L R. (ed.). Goldfrank's Toxicologic Emergencies. New York, New York: McGraw-Hill. pp. 1167.
- ^ Graham DY, Smith JL, Opekun AR. (1988). "Spicy food and the stomach. Evaluation by videoendoscopy.". JAMA 260 (23): 3473. doi:. PMID 3210286.
- ^ Myers BM, Smith JL, Graham DY (March 1987). "Effect of red pepper and black pepper on the stomach". Am. J. Gastroenterol. 82 (3): 211–4. PMID 3103424.
- ^ López-Carrillo L, López-Cervantes M, Robles-Díaz G, et al (2003). "Capsaicin consumption, Helicobacter pylori positivity and gastric cancer in Mexico". Int. J. Cancer 106 (2): 277–82. doi:. PMID 12800206.
- ^ Archer VE, Jones DW (October 2002). "Capsaicin pepper, cancer and ethnicity". Med. Hypotheses 59 (4): 450–7. doi:. PMID 12208187. http://linkinghub.elsevier.com/retrieve/pii/S0306987702001524.
[edit] General references
- Dray A (1992). "Mechanism of action of capsaicin-like molecules on sensory neurons". Life Sci. 51 (23): 1759–65. doi:. PMID 1331641.
- Garnanez RJ, McKee LH (2001) "Temporal effectiveness of sugar solutions on mouth burn by capsaicin" IFT Annual Meeting 2001
- Henkin R (November 1991). "Cooling the burn from hot peppers". JAMA 266 (19): 2766. doi:. PMID 1942431.
- Nasrawi CW, Pangborn RM (April 1990). "Temporal effectiveness of mouth-rinsing on capsaicin mouth-burn". Physiol. Behav. 47 (4): 617–23. doi:. PMID 2385629.
- Tewksbury JJ, Nabhan GP (July 2001). "Seed dispersal. Directed deterrence by capsaicin in chilies". Nature 412 (6845): 403–4. doi:. PMID 11473305.
- Kirifides ML, Kurnellas MP, Clark L, Bryant BP (February 2004). "Calcium responses of chicken trigeminal ganglion neurons to methyl anthranilate and capsaicin". J. Exp. Biol. 207 (Pt 5): 715–22. doi:. PMID 14747403. http://jeb.biologists.org/cgi/pmidlookup?view=long&pmid=14747403.
- Tarantula Venom, Chili Peppers Have Same "Bite," Study Finds http://news.nationalgeographic.com/news/2006/11/061108-tarantula-venom.html
[edit] See also
- TRPV1, the only known receptor (a transient receptor potential channel) for capsaicin.
- Piperine, the active piquant chemical in black pepper
- Allyl isothiocyanate, the active piquant chemical in mustard, radishes, horseradish, and wasabi
- Allicin, the active piquant flavor chemical in uncooked garlic and onions (see those articles for discussion of other chemicals in them relating to pungency, and eye irritation)
- Naga Jolokia pepper, the world's most capsaicin-rich fruit
[edit] External links
| Look up capsaicin in Wiktionary, the free dictionary. |
- Capsaicin Technical Fact Sheet - National Pesticide Information Center
- EPA Capsaicin Reregistration Eligibility Decision Fact Sheet
- Capsaicin and Its Therapeutic Potential
- Molecule of the Month
- The Magic of Chili Pepper and Capsaicin
- European Commission, opinion of the Scientific Committee on Food on capsaicin.
- A WikiHow article on How to Cool Chilli Pepper Burns.
- The Neurobiology of Disease wiki, from Connecticut College: Capsaicin.
|
|||||||||||||||||||


