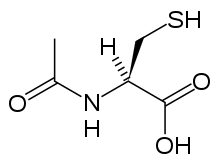
Acetylcysteine
From Wikipedia, the free encyclopedia
| This article needs additional citations for verification. Please help improve this article by adding reliable references (ideally, using inline citations). Unsourced material may be challenged and removed. (March 2009) |
 |
|
 |
|
|
Acetylcysteine
|
|
| Systematic (IUPAC) name | |
| (2R)-2-acetamido-3-sulfanylpropanoic acid | |
| Identifiers | |
| CAS number | |
| ATC code | R05 S01 V03 |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| Chemical data | |
| Formula | C5H9NO3S |
| Mol. mass | 163.19 |
| Synonyms | (R)-2-acetamido-3-mercaptopropanoic acid |
| Pharmacokinetic data | |
| Bioavailability | 6–10% (oral) <3% (topical) |
| Metabolism | hepatic |
| Half life | 5.6 hours (adults) 11 hours (neonates) |
| Excretion | renal |
| Therapeutic considerations | |
| Licence data | |
| Pregnancy cat. |
B2 (Aus) |
| Legal status | |
| Routes | inhalation, IV, oral |
Acetylcysteine (rINN; pronounced /ˌæsɛtəlˈsɪstiːn, əˌsɛtəl-/), also known as N-acetylcysteine or N-acetyl-L-cysteine (abbreviated NAC), is a pharmacological agent used mainly as a mucolytic agent and in the management of paracetamol (acetaminophen) overdose.
Contents |
[edit] Trade names
The drug is available under the trade names ASİST(Bilim Pharmaceuticals), ACC (Hexal AG), Mucomyst (Bristol-Myers Squibb), Acetadote (Cumberland Pharmaceuticals), Fluimucil (Zambon), Parvolex (GSK), Lysox (Menarini), Mucomelt-A tab (Venus Remedies, India), Mucinac (Cipla, India) Mucolysin (Sandoz) and Trebon N (Uni-pharma).
[edit] Dosage forms
Acetylcysteine is available in different dosage forms for different indications:
- Solution for inhalation (Mucomyst, Mucosil) – inhaled for mucolytic therapy or ingested for nephroprotective effect (to protect the kidneys)
- IV injection (Parvolex, Acetadote) – treatment of paracetamol/acetaminophen overdose
- Oral solution – various indications.
- Effervescent Tablets (200 mg) - Reolin (Hochland Pharma Germany) and Mucinac (Cipla India).
The IV injection and inhalation preparations are, in general, prescription only, whereas the oral solution and the effervescent tablets are available over the counter in many countries.
[edit] Clinical use
[edit] Mucolytic therapy
Inhaled acetylcysteine is indicated for mucolytic ("mucus-dissolving") therapy as an adjuvant in respiratory conditions with excessive and/or thick mucus production. Such conditions include emphysema, bronchitis, tuberculosis, bronchiectasis, amyloidosis, pneumonia. It is also used post-operatively, as a diagnostic aid, and in tracheotomy care. It may be considered ineffective in cystic fibrosis.[1] However, a recent paper in the Proceedings of the National Academy of Sciences reports that high-dose oral N-acetylcysteine modulates inflammation in cystic fibrosis and has the potential to counter the intertwined redox and inflammatory imbalances in CF.[2] Oral acetylcysteine may also be used as a mucolytic in less serious cases.
For this indication, acetylcysteine acts to reduce mucus viscosity by splitting disulfide bonds linking proteins present in the mucus (mucoproteins).
[edit] Paracetamol (Acetaminophen) overdose
Intravenous acetylcysteine is indicated for the treatment of paracetamol (acetaminophen) overdose. When paracetamol is taken in large quantities, a minor metabolite called N-acetyl-p-benzoquinone imine (NAPQI) builds up. It is normally conjugated by glutathione, but when taken in excess (especially in alcoholics), the body's glutathione reserves are not sufficient to inactivate the toxic NAPQI. This metabolite is then free to react with key hepatic enzymes, therefore damaging hepatocytes. This may lead to severe liver damage and even death by fulminant liver failure.
For this indication, acetylcysteine acts to augment the glutathione reserves in the body and, together with glutathione, directly bind to toxic metabolites. These actions serve to protect hepatocytes in the liver from NAPQI toxicity.
Although both IV and oral acetylcysteine are equally effective for this indication, oral administration is poorly tolerated, owing to the high doses required (due to low oral bioavailability,[3]) very unpleasant taste and odour, and adverse effects (particularly nausea and vomiting). Studies conducted by Baker and Dilger[4] suggest that the prior pharmacokinetic studies of N-acetylcysteine did not include Acetylation as a reason for the low bioavailability of N-acetylcysteine. In the research conducted by Baker,[4] it was concluded that oral N-acetylcysteine was identical in bioavailability to Cysteine precursors. (However, 3% to 6% of people given intravenous acetylcysteine show a severe, anaphylaxis-like allergic reaction, which may include extreme breathing difficulty (due to bronchospasm), a decrease in blood pressure, rash, angioedema, and sometimes also nausea and vomiting. [5] Repeated overdoses will cause the allergic reaction to progressively worsen.)
Several studies have found this anaphylaxis-like reaction to occur more often in people given IV acetylcysteine despite serum levels of paracetamol not high enough to be considered toxic. [6][7][8][9]
In some countries, a specific intravenous formulation does not exist to treat paracetamol overdose. In these cases, the formulation used for inhalation may be used intravenously.
[edit] Nephroprotective agent
Oral acetylcysteine is used for the prevention of radiocontrast-induced nephropathy (a form of acute renal failure). Some studies show that prior administration of acetylcysteine markedly decreases (90%) radiocontrast nephropathy,[10] whereas others appear to cast doubt on its efficacy.[11][12] Worth considering is the newest data published in two papers in the New England Journal of Medicine and the Journal of the American Medical Association. The authors' conclusions in those papers were:
- "Intravenous and oral N-acetylcysteine may prevent contrast-medium–induced nephropathy with a dose-dependent effect in patients treated with primary angioplasty and may improve hospital outcome."[13]
- "Acetylcysteine protects patients with moderate chronic renal insufficiency from contrast-induced deterioration in renal function after coronary angiographic procedures, with minimal adverse effects and at a low cost"[14]
Acetylcysteine continues to be commonly used in individuals with renal impairment to prevent the precipitation of acute renal failure.[citation needed]
[edit] Interstitial lung disease
Acetylcysteine is used in the treatment of interstitial lung disease to prevent disease progression.[citation needed]
[edit] Investigational
The following uses have not been well-established or investigated:
- NAC has been shown to reduce the symptoms of both schizophrenia[15] and bipolar disorder[16] in two placebo controlled trials.
- Evidence that NAC and other antioxidants can exert beneficial effects on pancreatic b-cell function in diabetes was published in a 1999 study. The authors conclude that a sufficient supply of antioxidants (NAC, vitamin C plus vitamin E, or both) may prevent or delay b-cell dysfunction in diabetes by providing protection against glucose toxicity.[17]
- NAC is undergoing clinical trials in the United States for the treatment of obsessive-compulsive disorder.[18] It is thought to counteract the glutamate hyperactivity in OCD.
- NAC has been shown to reduce cravings associated with chronic cocaine use in a study conducted at the Medical University of South Carolina[19][20]
- It may reduce the incidence of chronic obstructive pulmonary disease (COPD) exacerbations[21]
- In the treatment of AIDS, NAC has been shown to cause a "marked increase in immunological functions and plasma albumin concentrations"[22] Albumin concentration are inversely correlated with muscle wasting (cachexia), a condition associated with AIDS.
- An animal study indicates that acetylcysteine may decrease mortality associated with influenza [23]
- Animal studies suggest that NAC may help prevent noise-induced hearing loss. [24] A clinical trial to determine efficacy in preventing noise-induced sensorineural hearing loss in humans is currently (2006) being jointly conducted by the US Army and US Navy.[citation needed]
- It has been suggested that NAC may help sufferers of Samter's triad by increasing levels of glutathione allowing faster breakdown of salicylates, though there is no evidence that it is of benefit [25]
- There are claims that acetylcysteine taken together with vitamin C and B1 can be used to prevent and relieve symptoms of veisalgia (hangover following ethanol (alcohol) consumption). The claimed mechanism is through scavenging of acetaldehyde, a toxic intermediate in the metabolism of ethanol.[26][27]
- It has been shown to help women with PCOS (polycystic ovary syndrome) to reduce insulin problems and possibly improve fertility. [28]
- Small studies have shown acetylcysteine to be of benefit to sufferers of blepharitis[citation needed] and has been shown to reduce ocular soreness caused by Sjogren's syndrome.[citation needed]
- Studies in mice models of Ataxia Telangictasia (ATM knockout) indicate that NAC prevents genomic instability and retards lymphomagenesis in these animals.[citation needed] Clinical trials in human AT patients are underway.[citation needed]
[edit] Complexing agent
N-Acetylcysteine has been used to complex palladium, to help it dissolve in water. This helps to remove palladium from drugs or precursors synthesized by palladium-coupling reactions.[29]
[edit] Chemistry
Acetylcysteine is the N-acetyl derivative of the amino acid L-cysteine, and is a precursor in the formation of the antioxidant glutathione in the body. The thiol (sulfhydryl) group confers antioxidant effects and is able to reduce free radicals.
[edit] Possible toxicity
Researchers at the University of Virginia reported in 2007 study using very large doses in a mouse model that acetylcysteine, which is found in many bodybuilding supplements, could potentially cause damage to the heart and lungs.[30] They found that acetylcysteine was metabolized to S-nitroso-N-acetylcysteine (SNOAC), which increased blood pressure in the lungs and right ventricle of the heart (pulmonary artery hypertension) in mice treated with acetylcysteine. The effect was similar to that observed following a 3-week exposure to an oxygen-deprived environment (chronic hypoxia). The authors also found that SNOAC induced a hypoxia-like response in the expression of several important genes both in vitro and in vivo.
The implications of these findings for long-term treatment with acetylcysteine have not yet been investigated. The dose used by Palmer and colleagues was dramatically higher than that used in humans;[30] nonetheless, the drug's effects on the hypoxic ventilatory response have been observed previously in human subjects at more moderate doses.[31]
[edit] References
- ^ Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006.
- ^ Tirouvanziam R, Conrad CK, Bottiglieri T, Herzenberg LA, Moss RB, Herzenberg LA (2006). "High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis". PNAS 103 (12): 4628–33. doi:. PMID 16537378.
- ^ Borgström, L., B. Kågedal, and O. Paulsen (1986). "Pharmacokinetics of N-acetylcysteine 310 in man". Eur. J. Clin. Pharmacol. 31: 217–222. doi:.
- ^ a b Dilger, R. N., Baker, D. H. (2007). "Oral N-acetyl-L-cysteine is a safe and effective precursor of cysteine". Journal of Animal Science 85: 1712. doi:. PMID 17371789.
- ^ Kanter MZ (2006). "Comparison of oral and i.v. acetylcysteine in the treatment of acetaminophen poisoning". American Journal of Health-System Pharmacy 63: 1821. doi:. PMID 16990628. http://www.ajhp.org/cgi/content/full/63/19/1821.
- ^ Dawson AH, Henry DA, McEwen J (March 1989). "Adverse reactions to N-acetylcysteine during treatment for paracetamol poisoning". Med. J. Aust. 150 (6): 329–31. PMID 2716644.
- ^ Bailey B, McGuigan MA (June 1998). "Management of anaphylactoid reactions to intravenous N-acetylcysteine". Ann Emerg Med 31 (6): 710–5. PMID 9624310.
- ^ Schmidt LE, Dalhoff K (January 2001). "Risk factors in the development of adverse reactions to N-acetylcysteine in patients with paracetamol poisoning". Br J Clin Pharmacol 51 (1): 87–91. doi:. PMID 11167669.
- ^ Lynch RM, Robertson R (January 2004). "Anaphylactoid reactions to intravenous N-acetylcysteine: a prospective case controlled study". Accid Emerg Nurs 12 (1): 10–5. doi:. PMID 14700565.
- ^ Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W (July 2000). "Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine". N. Engl. J. Med. 343 (3): 180–4. PMID 10900277. http://content.nejm.org/cgi/content/full/343/3/180.
- ^ Hoffmann U, Fischereder M, Krüger B, Drobnik W, Krämer BK (February 2004). "The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable". J. Am. Soc. Nephrol. 15 (2): 407–10. PMID 14747387. http://jasn.asnjournals.org/cgi/pmidlookup?view=long&pmid=14747387.
- ^ Miner SE, Dzavik V, Nguyen-Ho P, et al (October 2004). "N-acetylcysteine reduces contrast-associated nephropathy but not clinical events during long-term follow-up". Am. Heart J. 148 (4): 690–5. doi:. PMID 15459602.
- ^ Marenzi G, Assanelli E, Marana I, et al (June 2006). "N-acetylcysteine and contrast-induced nephropathy in primary angioplasty". N. Engl. J. Med. 354 (26): 2773–82. doi:. PMID 16807414. http://content.nejm.org/cgi/content/full/354/26/2773.
- ^ Kay J, Chow WH, Chan TM, Lo SK, Kwok OH, Yip A, Fan K, Lee CH, Lam WF (2003). "Acetylcysteine for Prevention of Acute Deterioration of Renal Function Following Elective Coronary Angiography and Intervention: A Randomized Controlled Trial". JAMA the Journal of the American Medical Association 289: 553. doi:. PMID 12578487. http://jama.ama-assn.org/cgi/content/full/289/5/553.
- ^ Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, Ording-Jespersen S, Little J, Conus P, Cuenod M, Do KQ, Bush AI (September 2008). "N-acetyl cysteine as a glutathione precursor for schizophrenia--a double-blind, randomized, placebo-controlled trial". Biol. Psychiatry 64 (5): 361–8. doi:. PMID 18436195. http://linkinghub.elsevier.com/retrieve/pii/S0006-3223(08)00270-9.
- ^ Berk M, Copolov DL, Dean O, et al (September 2008). "N-acetyl cysteine for depressive symptoms in bipolar disorder--a double-blind randomized placebo-controlled trial". Biol. Psychiatry 64 (6): 468–75. doi:. PMID 18534556.
- ^ Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, et al (1999). "Beneficial Effects of Antioxidants in Diabetes: Possible Protection of Pancreatic b-cells Against Glucose Toxicity". Diabetes 48: 2398-2406. http://diabetes.diabetesjournals.org/cgi/reprint/48/12/2398.pdf.
- ^ N-Acetylcysteine Augmentation in Treatment-Refractory Obsessive-Compulsive Disorder - Full Text View - ClinicalTrials.gov
- ^ Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ (March 2007). "An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study". Prog. Neuropsychopharmacol. Biol. Psychiatry 31 (2): 389–94. doi:. PMID 17113207.
- ^ LaRowe SD, Myrick H, Hedden S, et al (July 2007). "Is cocaine desire reduced by N-acetylcysteine?". Am J Psychiatry 164 (7): 1115–7. doi:. PMID 17606664.
- ^ Pela R, Calcagni AM, Subiaco S, Isidori P, Tubaldi A, Sanguinetti CM (1999). "N-acetylcysteine reduces the exacerbation rate in patients with moderate to severe COPD". Respiration 66 (6): 495–500. PMID 10575333. http://content.karger.com/produktedb/produkte.asp?typ=fulltext&file=res66495.
- ^ Breitkreutz R, Pittack N, Nebe CT, et al (2000). "Improvement of immune functions in HIV infection by sulfur supplementation: two randomized trials". J. Mol. Med. 78 (1): 55–62. PMID 10759030.
- ^ Ungheri D, Pisani C, Sanson G, et al (2000). "Protective effect of N-acetylcysteine in a model of influenza infection in mice". Int J Immunopathol Pharmacol 13 (3): 123–128. PMID 12657201.
- ^ Kopke R, Bielefeld E, Liu J, et al (March 2005). "Prevention of impulse noise-induced hearing loss with antioxidants". Acta Otolaryngol. 125 (3): 235–43. PMID 15966690.
- ^ Bachert C, Hörmann K, Mösges R, et al (March 2003). "An update on the diagnosis and treatment of sinusitis and nasal polyposis". Allergy 58 (3): 176–91. doi:. PMID 12653791.
- ^ Fawkes, SW CERI: Living with Alcohol Smart Drug News 1996 Dec 13
- ^ Resat Ozaras, Veysel Tahan, Seval Aydin, Hafize Uzun, Safiye Kaya, Hakan Senturk. N-acetylcysteine attenuates alcohol-induced oxidative stess in rats World Journal of Gastroenterology 2003 Apr 15
- ^ Fulghesu AM, Ciampelli M, Muzj G, et al (June 2002). "N-acetyl-cysteine treatment improves insulin sensitivity in women with polycystic ovary syndrome". Fertil. Steril. 77 (6): 1128–35. doi:. PMID 12057717.
- ^ Garrett, Christine E., Christine E. Garrett; Kapa Prasad (2004). "The Art of Meeting Palladium Specifications in Active Pharmaceutical Ingredients Produced by Pd-Catalyzed Reactions". Advanced Synthesis & Catalysis 346 (8): 889–900. doi:.
- ^ a b Palmer LA, Doctor A, Chhabra P, et al (September 2007). "S-nitrosothiols signal hypoxia-mimetic vascular pathology". J. Clin. Invest. 117 (9): 2592–601. doi:. PMID 17786245.
- ^ Hildebrandt W, Alexander S, Bärtsch P, Dröge W (March 2002). "Effect of N-acetyl-cysteine on the hypoxic ventilatory response and erythropoietin production: linkage between plasma thiol redox state and O(2) chemosensitivity". Blood 99 (5): 1552–5. PMID 11861267. http://www.bloodjournal.org/cgi/pmidlookup?view=long&pmid=11861267.
- CIMS India database "[1]"
[edit] See also
[edit] External links
- MedlinePlus drug information: Acetylcysteine (inhalation) – information from USP DI Advice for the Patient
[edit] References
- British National Formulary 55, March 2008; ISBN 978 085369 776 3
|
||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

