Radiation
From Wikipedia, the free encyclopedia
In physics, radiation describes any process in which energy emitted by one body travels through a medium or through space, ultimately to be absorbed by another body. Non-physicists often associate the word with ionizing radiation (e.g., as occurring in nuclear weapons, nuclear reactors, and radioactive substances), but it can also refer to electromagnetic radiation (i.e., radio waves, infrared light, visible light, ultraviolet light, and X-rays) which can also be ionizing radiation, to acoustic radiation, or to other more obscure processes. What makes it radiation is that the energy radiates (i.e., it travels outward in straight lines in all directions) from the source. This geometry naturally leads to a system of measurements and physical units that are equally applicable to all types of radiation.
Contents |
[edit] Non-Ionizing Radiation
Non-ionizing (or non-ionising) radiation, by contrast, refers to any type of radiation that does not carry enough energy per quantum to ionize atoms or molecules. Most especially, it refers to the lower energy forms of electromagnetic radiation (i.e., radio waves, microwaves, terahertz radiation, infrared light, and visible light). The effects of these forms of radiation on living tissue have only recently been studied. Instead of producing charged ions when passing through matter, the electromagnetic radiation has sufficient energy only for excitation, the movement of an electron to a higher energy state. Nevertheless, different biological effects are observed for different types of non-ionizing radiation.[1][2]
[edit] Electromagnetic Radiation
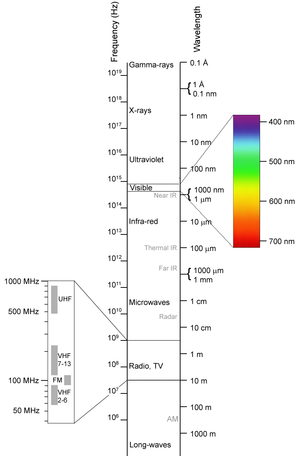
Electromagnetic radiation (sometimes abbreviated EMR) takes the form of self-propagating waves in a vacuum or in matter. EM radiation has an electric and magnetic field component which oscillate in phase perpendicular to each other and to the direction of energy propagation. Electromagnetic radiation is classified into types according to the frequency of the wave, these types include (in order of increasing frequency): radio waves, microwaves, terahertz radiation, infrared radiation, visible light, ultraviolet radiation, X-rays and gamma rays. Of these, radio waves have the longest wavelengths and Gamma rays have the shortest. A small window of frequencies, called visible spectrum or light, is sensed by the eye of various organisms, with variations of the limits of this narrow spectrum. EM radiation carries energy and momentum, which may be imparted when it interacts with matter.
[edit] Light
Light, or visible light, is electromagnetic radiation of a wavelength that is visible to the human eye (about 400–700 nm), or up to 380–750 nm.[1] In the broader field of physics, light is often used to refer to electromagnetic radiation of all wavelengths, whether visible or not.
[edit] Thermal Radiation
Thermal radiation is the process by which the surface of an object radiates its thermal energy in the form of electromagnetic waves. Infrared radiation from a common household radiator or electric heater is an example of thermal radiation, as is the light emitted by a glowing incandescent light bulb. Thermal radiation is generated when heat from the movement of charged particles within atoms is converted to electromagnetic radiation. The emitted wave frequency of the thermal radiation is a probability distribution depending only on temperature, and for a genuine black body is given by Planck’s law of radiation. Wien's law gives the most likely frequency of the emitted radiation, and the Stefan–Boltzmann law gives the heat intensity.
[edit] Black Body Radiation
Black Body Radiation is a common synonym for thermal radiation (see above). It is so-called because the ideal radiator of thermal energy would also be an ideal absorber of thermal energy: It would not reflect any light, and thus would appear to be absolutely black.
[edit] Discovery
Wilhelm Röntgen is credited with the discovery of X-Rays. When experimenting with various isotopes of tritium, he noticed a drastic change in photonic emissions when measuring electrical charges in a vacuum. When he took pictures of the tritium, he found that the state of one solid piece would deteriorate quickly. Henri Becquerel found that uranium salts caused fogging of an unexposed photographic plate, and Marie Curie discovered that only certain elements gave off these rays of energy. She named this behaviour radioactivity.
In December 1899, Marie Curie and Pierre Curie discovered radium in pitchblende. This new element was two million times more radioactive than uranium, as described by Marie.
[edit] The Electromagnetic Spectrum
The electromagnetic (EM) spectrum is the range of all possible electromagnetic radiation frequencies.[1] The "electromagnetic spectrum" (usually just spectrum) of an object is the characteristic distribution of electromagnetic radiation from that particular object.
[edit] See also
- Ionizing radiation
- Non-ionizing radiation
- Background radiation, which actually refers to the background ionizing radiation
- Black hole
- Cosmic microwave background radiation, 3K blackbody radiation that fills the Universe
- Electromagnetic spectrum
- Radiant energy, radiation by a source into the surrounding environment.
- Radiation damage - adverse effects on materials and devices
- Radiation hormesis - dosage threshold damage theory
- Radiation poisoning - adverse effects on life forms
- Radiation hardening - making devices resistant to failure in high radiation environments
- Radioactive contamination
- Radioactive decay
- Hawking radiation
- Cherenkov radiation
- Cyclotron radiation
- Solar radiation
- Synchrotron radiation
- Radiation reaction
- National Council on Radiation Protection and Measurements
[edit] References
- ^ Kwan-Hoong Ng (20th – 22nd October 2003). "[http://www.who.int/peh-emf/meetings/archive/en/keynote3ng.pdf Non-Ionizing Radiations – Sources, Biological Effects, Emissions and Exposures]" (PDF). Proceedings of the International Conference on Non-Ionizing Radiation at UNITEN ICNIR2003 Electromagnetic Fields and Our Health. http://www.who.int/peh-emf/meetings/archive/en/keynote3ng.pdf.
- ^ John E. Moulder. "Static Electric and Magnetic Fields and Human Health". http://www.mcw.edu/gcrc/cop/static-fields-cancer-FAQ/toc.html.