Sodium acetate
From Wikipedia, the free encyclopedia
| Sodium acetate | |
|---|---|
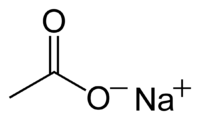 |
|
| IUPAC name |
|
| Other names | sodium salt |
| Identifiers | |
| ChemSpider ID | |
| Properties | |
| Molecular formula | NaCH3COO |
| Molar mass | 82.03 g/mol (anhydrous) 136.08 g/mol (trihydrate) |
| Appearance | White deliquescent powder |
| Density | 1.45 g/cm³, solid |
| Melting point |
Decomposes at 324 °C |
| Boiling point |
Decomposes |
| Solubility in water | 76 g/100 ml (0°C) |
| Basicity (pKb) | 9.25 |
| Structure | |
| Crystal structure | monoclinic |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Irritant |
| NFPA 704 | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Sodium acetate, (also sodium ethanoate) is the sodium salt of acetic acid. It is an inexpensive chemical produced in industrial quantities for a wide range of uses.
Contents |
[edit] Applications
[edit] Industrial
Sodium acetate is used in the textile industry to neutralize sulfuric acid waste streams, and as a photoresist while using aniline dyes. It is also a pickling agent in chrome tanning, and it helps to retard vulcanization of chloroprene in synthetic rubber production.
[edit] Food
Sodium acetate may be added to foods as a seasoning, and to alcoholic beverages to decrease the risk of a hangover. It may be used in the form of sodium diacetate — a 1:1 complex of sodium acetate and acetic acid,[1] given the E-number E262.
[edit] Buffer solution
As the conjugate base of a weak acid, a solution of sodium acetate and acetic acid can act as a buffer to keep a relatively constant pH. This is useful especially in biochemical applications where reactions are pH dependent.
[edit] Heating pad

Sodium acetate is also used in consumer heating pads or hand warmers and is also used in hot ice. Sodium acetate trihydrate crystals melt at 58 °C, dissolving in their water of crystallization. When they are heated to around 100 °C, and subsequently allowed to cool, the aqueous solution becomes supersaturated. This solution is capable of supercooling to room temperature without forming crystals. By clicking on a metal disc in the heating pad, a nucleation center is formed which causes the solution to crystallize into solid sodium acetate trihydrate again. The bond-forming process of crystallization is exothermic, hence heat is emitted.[2][3][4] The latent heat of fusion is about 264–289 kJ/kg.[5] Unlike some other types of heat packs that depend on irreversible chemical reactions, sodium acetate heat packs can be easily recharged by boiling until all crystals are dissolved. Therefore they can be recycled indefinitely.[6]
[edit] Preparation
Sodium acetate is inexpensive, and is usually purchased from chemical suppliers, instead of being synthesized in the laboratory. It is sometimes produced in a laboratory experiment by the reaction of acetic acid with sodium carbonate, sodium bicarbonate, or sodium hydroxide. These reactions produce aqueous sodium acetate, and water. Carbon dioxide is produced in the reaction with sodium carbonate and bicarbonate, and it leaves the reaction vessel as a gas (unless the reaction vessel is pressurized).
- CH3–COOH + Na+[HCO3]– → CH3–COO– Na+ + H2O + CO2
This is the well-known "fizzing" reaction between baking soda and vinegar. 84 grams of sodium bicarbonate (baking soda) react with 750 ml of 8% vinegar to make 82 g sodium acetate in water. By boiling off most of the water, one can refine either a concentrated solution of sodium acetate or crystals.
[edit] Reactions
Sodium acetate can be used to form an ester with an alkyl halide such as bromoethane:
- H3C–COO– Na+ + Br–CH2–CH3 → H3C–COO–CH2–CH3 + NaBr
In such a reaction, the sodium acetate is usually complexed with caesium in order to increase the nucleophilicity of the carboxylate group.
[edit] References
- ^ http://www.jungbunzlauer.com/products-applications/products/specialties/sodium-diacetate/general-information.html
- ^ "Crystallization of Supersaturated Sodium Acetate". Journal of Chemical Education. http://jchemed.chem.wisc.edu/JCESoft/CCA/CCA3/MAIN/ACETATE/PAGE1.HTM.
- ^ Fake latent heat and supersaturation
- ^ "How do sodium acetate heat pads work?". HowStuffWorks. http://www.howstuffworks.com/question290.htm. Retrieved on 2007-09-03.
- ^ Thermal Energy Storage: Systems and Applications, By Ibrahim Dincer, Marc A. Rosen, p. 155 [1]
- ^ "Infinite Activations". Cloud Depot Nine. http://www.clouddepotnine.com/index.html.
[edit] External links
- Hot Ice – Instructions, Pictures, and Videos
- Video on how to make hot ice using sodium acetate
- More information, videos, and pictures
|
|||||



