Functional group
From Wikipedia, the free encyclopedia

In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a part of.[1][2] However, its relative reactivity can be modified by nearby functional groups.
The word moiety is often used synonymously to "functional group", but according to the IUPAC definition,[citation needed] a moiety is a half of a molecule including substructures of functional groups. For example, an ester is divided into an alcohol and an acyl moiety, but has an ester functional group.
Combining the names of functional groups with the names of the parent alkanes generates a powerful systematic nomenclature for naming organic compounds.
The non-hydrogen atoms of functional groups are always associated with each other and with the rest of the molecule by covalent bonds. When the group of atoms is associated with the rest of the molecule primarily by ionic forces, the group is referred to more properly as a polyatomic ion or complex ion. And all of these are called radicals, by a meaning of the term radical that predates the free radical.
The first carbon atom after the carbon that attaches to the functional group is called the alpha carbon; the second, beta carbon, the third, gamma carbon, etc. If there is another functional group at a carbon, it may be named with the Greek letter, e.g. the gamma-amine in gamma-aminobutanoic acid is on the third carbon of the carbon chain attached to the carboxylic acid group.
Contents |
[edit] Synthetic chemistry
Organic reactions are facilitated and controlled by the functional groups of the reactants. Alkyls are generally unreactive and difficult to get to react selectively at the desired positions, with few exceptions. In contrast, unsaturated carbon functional groups, and carbon-oxygen and carbon-nitrogen functional groups have a more diverse array of reactions that are also selective. It may be necessary to create a functional group in the molecule to make it react. For example, to synthesize iso-octane (8-carbon) from the unfunctionalized alkane isobutane (4-carbon), isobutane is first dehydrogenated into isobutene. This contains the alkene functional group and can now dimerize with another isobutene to give iso-octene, which is hydrogenated to octane.
[edit] Functionalization
Functionalization is the addition of functional groups onto the surface of a material by chemical synthesis methods. The functional group added can be subjected to ordinary synthesis methods to attach virtually any kind of organic compound onto the surface.
Functionalization is employed for surface modification of industrial materials in order to achieve desired surface properties such as water repellent coatings for automobile windshields and non-biofouling, hydrophilic coatings for contact lenses. Additionally, functional groups are used to covalently link functional molecules to the surface of chemical and biochemical devices such as microarrays and microelectromechanical systems.
Catalysts can be attached to a material that has been functionalized. For example, silica is functionalized with an alkyl silicone, where the alkyl contains an amine functional group. A ligand such as an EDTA fragment is synthesized onto the amine, and a metal cation is complexed into the EDTA fragment. The EDTA is not adsorbed onto the surface, but connected by a permanent chemical bond.
Functional groups are also used to covalently link molecules such as fluorescent dyes, nanoparticles, proteins, DNA, and other compounds of interest for a variety of applications such as sensing and basic chemical research.
[edit] Table of common functional groups
The following is a list of common functional groups. In the formulas, the symbols R and R' usually denote an attached hydrogen, or a hydrocarbon side chain of any length, but may sometimes refer to any group of atoms.
[edit] Hydrocarbons
Functional groups that vary based upon the number and order of π bonds impart different chemistry. Each listing below contains C-H bonds, but each one differs in type (and scope) of reactivity.
| Chemical class | Group | Formula | Structural Formula | Prefix | Suffix | Example |
|---|---|---|---|---|---|---|
| Alkane | Alkyl | RH | alkyl- | -ane |  Ethane |
|
| Alkene | Alkenyl | R2C=CR2 |  |
alkenyl- | -ene | 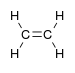 Ethylene (Ethene) |
| Alkyne | Alkynyl | RC≡CR' | alkynyl- | -yne | Acetylene (Ethyne) |
|
| Benzene derivative | Phenyl | RC6H5 RPh |
phenyl- | -benzene | 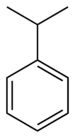 Cumene (2-phenylpropane) |
|
| Toluene derivative | Benzyl | RCH2C6H5 RBn |
benzyl- | 1-(substituent)toluene | Benzyl bromide (1-Bromotoluene) |
There are also a large number of branched or ring alkanes that have specific names, e.g. tert-butyl, bornyl, cyclohexyl, etc.
[edit] Groups containing halogens
Haloalkanes are a class of molecule that is defined by a carbon-halogen bond. This bond can be relatively weak (in the case of an iodoalkane) or quite stable (as in the case of a fluoroalkane). In general, with the exception of fluorinated compounds, haloalkanes readily undergo nucleophilic substitution reactions or elimination reactions. The substitution on the carbon, the acidity of an adjacent proton, the solvent conditions, etc. all can influence the outcome of the reactivity.
| Chemical class | Group | Formula | Structural Formula | Prefix | Suffix | Example |
|---|---|---|---|---|---|---|
| haloalkane | halo | RX | halo- | alkyl halide | Chloroethane (Ethyl chloride) |
|
| fluoroalkane | fluoro | RF | fluoro- | alkyl fluoride |  Fluoromethane (Methyl fluoride) |
|
| chloroalkane | chloro | RCl | chloro- | alkyl chloride | 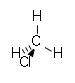 Chloromethane (Methyl chloride) |
|
| bromoalkane | bromo | RBr | bromo- | alkyl bromide |  Bromomethane (Methyl bromide) |
|
| iodoalkane | iodo | RI | iodo- | alkyl iodide |  Iodomethane (Methyl iodide) |
[edit] Groups containing oxygen
Compounds that contain C-O bonds each possess differing reactivity based upon the location and hybridization of the C-O bond, owing to the electron-withdrawing effect of sp² hybridized oxygen and the donating effects of sp³ hybridized oxygen.
| Chemical class | Group | Formula | Structural Formula | Prefix | Suffix | Example |
|---|---|---|---|---|---|---|
| Acyl halide | Haloformyl | RCOX | 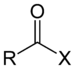 |
haloformyl- | -oyl halide |  Acetyl chloride (Ethanoyl chloride) |
| Alcohol | Hydroxyl | ROH |  |
hydroxy- | -ol | 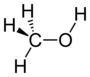 Methanol |
| Ketone | Carbonyl | RCOR' | 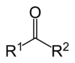 |
keto-, oxo- | -one | 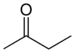 Methyl ethyl ketone (Butanone) |
| Aldehyde | Aldehyde | RCHO |  |
aldo- | -al |  Acetaldehyde (Ethanal) |
| Carbonate | Carbonate ester | ROCOOR | alkyl carbonate | |||
| Carboxylate | Carboxylate | RCOO− | 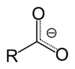 |
carboxy- | -oate | Sodium acetate (Sodium ethanoate) |
| Carboxylic acid | Carboxyl | RCOOH | 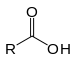 |
carboxy- | -oic acid |  Acetic acid (Ethanoic acid) |
| Ether | Ether | ROR' | alkoxy- | alkyl alkyl ether | Diethyl ether (Ethoxyethane) |
|
| Ester | Ester | RCOOR' |  |
alkyl alkanoate | Ethyl butyrate (Ethyl butanoate) |
|
| Hydroperoxide | Hydroperoxy | ROOH | hydroperoxy- | alkyl hydroperoxide | Methyl ethyl ketone peroxide |
|
| Peroxide | Peroxy | ROOR |  |
peroxy- | alkyl peroxide | Di-tert-butyl peroxide |
[edit] Groups containing nitrogen
Compounds that contain Nitrogen in this category may contain C-O bonds, such as in the case of amides.
| Chemical class | Group | Formula | Structural Formula | Prefix | Suffix | Example |
|---|---|---|---|---|---|---|
| Amide | Carboxamide | RCONR2 |  |
carboxamido- | -amide |  Acetamide (Ethanamide) |
| Amines | Primary amine | RNH2 | amino- | -amine | 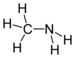 Methylamine (Methanamine) |
|
| Secondary amine | R2NH |  |
amino- | -amine | Dimethylamine |
|
| Tertiary amine | R3N |  |
amino- | -amine |  Trimethylamine |
|
| 4° ammonium ion | R4N+ |  |
ammonio- | -ammonium | 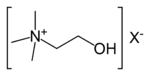 Choline |
|
| Imine | Primary ketimine | RC(=NH)R' | 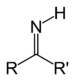 |
imino- | -imine | |
| Secondary ketimine | RC(=NR)R' |  |
imino- | -imine | ||
| Primary aldimine | RC(=NH)H | 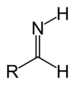 |
imino- | -imine | ||
| Secondary aldimine | RC(=NR')H |  |
imino- | -imine | ||
| Imide | Imide | RC(=O)NC(=O)R' |  |
imido- | -imide | |
| Azide | Azide | RN3 | azido- | alkyl azide |  Phenyl azide (Azidobenzene) |
|
| Azo compound | Azo (Diimide) |
RN2R' | 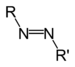 |
azo- | -diazene | 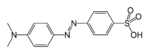 Methyl orange (p-dimethylamino-azobenzenesulfonic acid) |
| Cyanates | Cyanate | ROCN | cyanato- | alkyl cyanate | 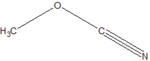 |
|
| Isocyanide | RNC | isocyano- | alkyl isocyanide | |||
| Isocyanates | Isocyanate | RNCO | isocyanato- | alkyl isocyanate | Methyl isocyanate |
|
| Isothiocyanate | RNCS | isothiocyanato- | alkyl isothiocyanate | Allyl isothiocyanate |
||
| Nitrate | Nitrate | RONO2 | nitrooxy-, nitroxy- |
alkyl nitrate |
 Amyl nitrate (1-nitrooxypentane) |
|
| Nitrile | Nitrile | RCN | cyano- |
alkanenitrile |
 Benzonitrile (Phenyl cyanide) |
|
| Nitrite | Nitrosooxy | RONO | nitrosooxy- |
alkyl nitrite |
 Isoamyl nitrite (3-methyl-1-nitrosooxybutane) |
|
| Nitro compound | Nitro | RNO2 |  |
nitro- | 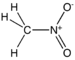 Nitromethane |
|
| Nitroso compound | Nitroso | RNO | nitroso- | Nitrosobenzene |
||
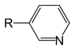
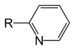
| Pyridine derivative | Pyridyl | RC5H4N |
4-pyridyl 3-pyridyl 2-pyridyl |
-pyridine |  Nicotine |
[edit] Groups containing phosphorus and sulfur
Compounds that contain sulfur and phosphorus exhibit unique chemistry due to their ability to form more bonds than nitrogen and oxygen, their lighter analogues on the periodic table.
| Chemical class | Group | Formula | Structural Formula | Prefix | Suffix | Example |
|---|---|---|---|---|---|---|
| Phosphine | Phosphino | R3P | 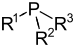 |
phosphino- | -phosphane | Methylpropylphosphane |
| Phosphodiester | Phosphate | HOPO(OR)2 | 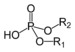 |
phosphoric acid di(substituent) ester | di(substituent) hydrogenphosphate | DNA |
| Phosphonic acid | Phosphono | RP(=O)(OH)2 |  |
phosphono- | substituent phosphonic acid | Benzylphosphonic acid |
| Phosphate | Phosphate | ROP(=O)(OH)2 |  |
phospho- | Glyceraldehyde 3-phosphate |
|
| Sulfide or thioether | RSR' | di(substituent) sulfide | Dimethyl sulfide |
|||
| Sulfone | Sulfonyl | RSO2R' | 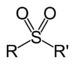 |
sulfonyl- | di(substituent) sulfone |  Dimethyl sulfone (Methylsulfonylmethane) |
| Sulfonic acid | Sulfo | RSO3H | 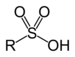 |
sulfo- | substituent sulfonic acid |  Benzenesulfonic acid |
| Sulfoxide | Sulfinyl | RSOR' | 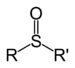 |
sulfinyl- | di(substituent) sulfoxide | 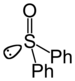 Diphenyl sulfoxide |
| Thiol | Sulfhydryl | RSH |  |
mercapto-, sulfanyl- | -thiol | Ethanethiol (Ethyl mercaptan) |
| Thiocyanate | Thiocyanate | RSCN | thiocyanato- | alkyl thiocyanate | ||
| Disulfide | Disulfide | RSSR' | alkyl alkyl disulfide |  Diphenyl disulfide 1,2-diphenyldisulfane |
[edit] Other
- For a list of all functional groups: Category:Functional groups
[edit] References
- ^ Compendium of Chemical Terminology (IUPAC "Gold Book") http://goldbook.iupac.org/F02555.html
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
[edit] External links
- IUPAC Blue Book (organic nomenclature)
- IUPAC ligand abbreviations (pdf)
- Organic Chemistry Help: Functional Groups Flashcards
|
|||||
|
|||||