Significant figures
From Wikipedia, the free encyclopedia
The significant figures (also called significant digits and abbreviated sig figs, sign.figs or sig digs) of a number are those digits that carry meaning contributing to its precision (see entry for Accuracy and precision). This includes all digits except:
- leading and trailing zeros (unless a decimal point is present) where they serve merely as placeholders to indicate the scale of the number.
- spurious digits introduced, for example, by calculations carried out to greater accuracy than that of the original data, or measurements reported to a greater precision than the equipment supports.
The concept of significant figures is often used in connection with rounding. Rounding to n significant figures is a more general-purpose technique than rounding to n decimal places, since it handles numbers of different scales in a uniform way. A practical calculation that uses any irrational number necessitates rounding the number, and hence the answer, to a finite number of significant figures. Computer representations of floating point numbers typically use a form of rounding to significant figures, but with binary numbers.
The term "significant figures" can also refer to a crude form of error representation based around significant figure rounding; for this use, see Significance arithmetic.
Contents |
[edit] Identifying significant digits
The rules for identifying significant digits when writing or interpreting numbers are as follows:
- All non-zero digits are considered significant. Example: 1, 20, and 300 all have one significant figure. Their significant figures are 1, 2, and 3 respectively. 123.45 has five significant figures: 1, 2, 3, 4 and 5.
- Zeros appearing anywhere between two non-zero digits are significant. Example: 101.12 has five significant figures: 1, 0, 1, 1 and 2.
- Leading zeros are not significant. For example, 0.00012 has two significant figures: 1 and 2.
- Trailing zeros in a number containing a decimal point are significant. For example, 12.2300 has six significant figures: 1, 2, 2, 3, 0 and 0. The number 0.000122300 still has only six significant figures (the zeros before the 1 are not significant). In addition, 120.00 has five significant figures. This convention clarifies the precision of such numbers; for example, if a result accurate to four decimal places is given as 12.23 then it might be understood that only two decimal places of accuracy are available. Stating the result as 12.2300 makes clear that it is accurate to four decimal places.
- The significance of trailing zeros in a number not containing a decimal point can be ambiguous. For example, it may not always be clear if a number like 1300 is accurate to the nearest unit (and just happens coincidentally to be an exact multiple of a hundred) or if it is only shown to the nearest hundred due to rounding or uncertainty. Various conventions exist to address this issue:
-
- A bar may be placed over the last significant digit; any trailing zeros following this are insignificant. For example,
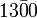 has three significant figures (and hence indicates that the number is accurate to the nearest ten).
has three significant figures (and hence indicates that the number is accurate to the nearest ten).
- A bar may be placed over the last significant digit; any trailing zeros following this are insignificant. For example,
-
- The last significant figure of a number may be underlined; for example, "20000" has two significant figures.
-
- A decimal point may be placed after the number; for example "100." indicates specifically that three significant figures are meant.[1]
- However, these conventions are not universally used, and it is often necessary to determine from context whether such trailing zeros are intended to be significant. If all else fails, the level of rounding can be specified explicitly. The abbreviation s.f. is sometimes used, for example "20 000 to 2 s.f." or "20 000 (2 sf)". Alternatively, the uncertainty can be stated separately and explicitly, as in 20 000 ± 1%, so that significant-figures rules do not apply.
A number with all zero digits (e.g. 0.000) has no significant digits, because the uncertainty is larger than the actual measurement.
[edit] Scientific notation
Generally, the same rules apply to numbers expressed in scientific notation. However, in the normalized form of that notation, placeholder leading and trailing digits do not occur, so all digits are significant. For example, 0.00012 (two significant figures) becomes 1.2×10−4, and 0.000122300 (six significant figures) becomes 1.22300×10−4. In particular, the potential ambiguity about the significance of trailing zeros is eliminated. For example, 0.1300 (four significant figures) is written as 1.300×10-1, while 1300 (two significant figures) is written as 1.3×103.
[edit] Rounding
To round to n significant figures:
- Start with the leftmost non-zero digit (e.g. the '1' in 1200, or the '2' in 0.0256).
- Keep n digits. Replace the rest with zeros.
- Round up by one if appropriate. For example, if rounding 0.039 to 1 significant figure, the result would be 0.04. There are several different rules for handling borderline cases — see rounding for more details.
[edit] Arithmetic
For multiplication and division, the result should have as many significant figures as the measured number with the smallest number of significant figures.
For addition and subtraction, the result should have as many decimal places as the measured number with the smallest number of decimal places.
[edit] Importance
[edit] Superfluous precision
If a sprinter is measured to have completed a 100 m race in 11.71 seconds, what is the sprinter's average speed? By dividing the distance by the time using a calculator, we get a speed of 8.53970965 m/s.
The most straightforward way to indicate the precision of this result (or any result) is to state the uncertainty separately and explicitly, for example as 8.540±0.085 m/s or equivalently 8.540(85) m/s. This is particularly appropriate when the uncertainty itself is important and precisely known. In this case, it is safe and indeed advantageous to provide more digits than would be called for by the significant-figures rules.
If the degree of precision in the answer is not important, it is again safe to express trailing digits that are not known exactly, for example 8.5397 m/s.
If, however, we are forced to apply significant-figures rules, expressing the result as 8.53970965 m/s would seem to imply that the speed is known to the nearest 10 nm/s or thereabouts, which would improperly overstate the precision of the measurement. Reporting the result using three significant figures (8.54 m/s) might be interpreted as implying that the speed is somewhere between 8.535 and 8.545 m/s. This again overstates the accuracy, but not nearly so badly. Reporting the result using two significant figures (8.5 m/s) would introduce considerable roundoff error and degrade the precision of the result.
[edit] Comprehension
Numbers are often rounded off to make them easier to read. It's easier for someone to compare (say) 18% to 36% than to compare 18.148% to 35.922%. Similarly, when reviewing a budget, a series of figures like:
Division A: $185 000 Division B: $ 45 000 Division C: $ 67 000
is easier to understand and compare than a series like:
Division A: $184 982 Division B: $ 44 689 Division C: $ 67 422
To reduce ambiguity, such data are sometimes represented to the nearest order of magnitude, like:
Revenue (in thousands of dollars): Division A: 185 Division B: 45 Division C: 67
[edit] Ineffectiveness
People who are not experts in metrology or statistics can overestimate the usefulness of significant figures. The topic receives much more emphasis in high-school and undergraduate chemistry texts[2] [1] than it does in real-world research laboratories.[3] [4]
Practicing scientists commonly express uncertain quantities in the form 1.23±0.06 or equivalently 1.23(6). The benefit is that the nominal value of the quantity is expressed by one numeral (1.23) while the uncertainty is expressed by a separate numeral (0.06). Expressing these two things explicitly and separately is more sensible than trying to encode both the nominal value and the uncertainty into a single numeral, where the uncertaintly range is constrained to being a power of ten.
"Significant figures" primarily refers to a type of rounding, and is arguably appropriate when roundoff of the final answer is the dominant contribution to the uncertainty. However, there are many important situations where roundoff of the final answer is not the dominant contribution to the uncertainty. Indeed, in experimental research (especially metrology), only in a very badly designed experiment would such roundoff error be dominant, because roundoff errors are so easily reduced. Furthermore, even when roundoff error is dominant, it is preferable to indicate this explicitly, as in 1.24(½) or equivalently 1.24(⁄).
Secondarily, "significant figures" may refer to a crude scheme for significance arithmetic, but as discussed in the significance arithmetic article and elsewhere,[5] there is generally not any rigorous way to express the uncertainty using significant figures.
In computer science and numerical analysis, good practice demands the use of guard digits. This is incompatible with any notion of significant figures. For a discussion, see Acton.[6]
Good examples of how real scientists express uncertain quantities can be found in the NIST compendium of physical constants.[3] None of the values there conform to any "significant figures" rules.
Procedures for how to properly represent uncertainty, and the rationale for these procedures, can be found in the references.[5] [4]
[edit] Most significant, least significant
In binary notation, it is common to refer to most or least significant digits to refer to the left-most or right-most digits in the binary string. For example in the binary string:
1010 1111
the most significant are 1010 and the least significant are 1111.
[edit] See also
[edit] References
- ^ a b Myers, R. Thomas; Oldham, Keith B.; Tocci, Salvatore.. "2" (Textbook). Chemistry. Austin, Texas: Holt Rinehart Winston. p. 59. ISBN 0-03-052002-9.
- ^ Bursten, Bruce Edward; Brown, Theodore; LeMay, Harold Eugene (1991). Chemistry : the central science. Englewood Cliffs (New Jersey): : Prentice Hall. ISBN 0-13-126202-5.
- ^ a b NIST compendium of physical constants
- ^ a b The NIST Reference on Constants, Units and Uncertainty : Uncertainty of Measurement Results
- ^ a b Measurements and Uncertainties
- ^ Acton, Forman (Textbook). Numerical Methods That Work. The Mathematical Association of America.
[edit] Additional reading
- ASTM E29-06b, Standard Practice for Using Significant Digits in Test Data to Determine Conformance with Specifications

