Conjugated linoleic acid
From Wikipedia, the free encyclopedia
| c9, t11 conjugated linoleic acid | |
|---|---|
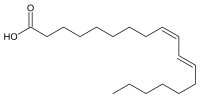 |
|
| IUPAC name |
|
| Other names | Bovinic acid, Rumenic acid |
| Identifiers | |
| CAS number | 2420-56-6 |
| PubChem | |
| SMILES |
|
| Properties | |
| Molecular formula | C18H32O2 |
| Molar mass | 280.44548 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Conjugated linoleic acids (CLA) are a family of at least 28[1] isomers of linoleic acid found especially in the meat and dairy products derived from ruminants. As the name implies, the double bonds of CLAs are conjugated.
Contents |
[edit] History
CLAs were discovered accidentally by researchers looking for mutagens in beef. In 1979, researchers from the University of Wisconsin applied a beef extract to mice skin. The mice were then exposed to a strong carcinogen (isolated from tobacco). When the researchers counted the number of tumors developed by the mice 16 weeks later, they found, to their surprise, that the mice exposed to the beef extract had 20 % fewer tumors. The identity of this anti-carcinogen was not discovered till almost a decade later in 1987. Micheal Pariza, the scientist who discovered CLA, later remarked that "few anticarcinogens, and certainly no other known fatty acids, are as effective as CLA in inhibiting carcinogenesis in these models." [2] [3]
[edit] Biochemistry
Most studies of CLAs have used a mixture of isomers wherein the isomers c9, t11-CLA and t10, c12-CLA were the most abundant ones.[4]
Conjugated linoleic acid is both a trans fatty acid and a cis fatty acid. The cis bond causes a lower melting point and ostensibly also the observed beneficial health effects. Unlike other trans fatty acids, it is not harmful, but beneficial.[5] CLA is conjugated, and in the United States, trans linkages in a conjugated system are not counted as trans fats for the purposes of nutritional regulations and labeling. CLA and some trans isomers of oleic acid are produced by microorganisms in the rumens of ruminants. Non-ruminants, including humans, produce certain isomers of CLA from trans isomers of oleic acid, such as vaccenic acid, which is converted to CLA by delta-9-desaturase.[6][7]
[edit] Diet and health
Antioxidant and anti-cancer properties have been attributed to CLA, and studies on mice and rats show encouraging results in hindering the growth of tumors in mammary, skin, and colon tissues.[8][9][10][11][12][13][14][15][16] [17] [18][19][20][21][22] It has been reported that CLA can up-regulate the tumor suppressor gene PTPRG, and may have anti cancer properties.[23][9]
A European team led by the Swiss scientist Lukas Rist has found that mothers consuming mostly organic milk and meat products have about 50 percent higher levels of rumenic acid in their breast milk.[24]
Some studies of CLA in human diets show that it may reduce body fat,[25] , especially abdominal fat. A maximum reduction in body fat was achieved with a daily dose of 3.4g.[26] However,experts do not recommend taking CLA supplements. CLA supplements contain high levels of the t10,c12 CLA isomer, which has been linked to multiple side effects.[27] Supplementation with this form of CLA has been shown to increase C-reactive protein levels, possibly to induce oxidative stress,[28] to reduce insulin sensitivity, and to increase lipid peroxidation.[29] However, the significance of these findings is unknown, and other studies suggest that CLA may protect cells from oxidative damage by increasing glutathione levels without inducing lipid peroxidation.[30] It is possible, however, that the observation of markers of increased lipid oxidation may indicate potentially desirable lipolytic effects. Further studies are necessary to establish the clinical significance of such observations.
[edit] Possible adverse effects of CLA in humans
There are concerns that the use of CLA by overweight people may tend to cause or to aggravate insulin resistance, which may increase their risk of developing diabetes.[31][32] However, the evidence is controversial, and some studies showed no changes in insulin sensitivity.[33][34]
In one study CLA produced a 32% increase in biliary cholesterol concentration which increases the chance of gallstone formation.[35]
In 2006, a study by the US Department of Agriculture suggested that CLA can induce essential fatty acid redistribution in mice. Changes in docosahexaenoic acid (DHA) and arachidonic acid (AA) levels were observed in some organs. For instance, certain CLA isomers reduced the DHA content of heart tissue by 25%, while in the spleen, DHA content rose, and AA fell.[4] A study of CLA supplementation in hatchling chicks (2005) showed high mortality and low hatchability rates among CLA-supplemented groups, and also a decrease in brain DHA levels of CLA-treated chicks[1]. These studies raise the question of whether CLA may increase the risk of cardiovascular and inflammatory diseases, but it has yet to be established whether such changes occur in humans, and whether they are clinically relevant.
[edit] Dietary Sources
Of all foods, kangaroo meat may have the highest concentration of CLA.[36] Food products (e.g. mutton and beef) from grass-fed ruminants are good sources of CLA, and contain much more of it than those from grain-fed animals.[37] In fact, meat and dairy products from grass-fed animals can produce 300-500% more CLA than those of cattle fed the usual diet of 50% hay and silage, and 50% grain.[38]
Eggs are also rich in CLA, and it has been shown that CLA in eggs survives the temperatures encountered during frying.[39]
Some mushrooms like Agaricus bisporus, are a rare vegetable source of CLA.[40]
[edit] See also
[edit] References
- ^ Banni S (June 2002). "Conjugated linoleic acid metabolism". Curr. Opin. Lipidol. 13 (3): 261–6. PMID 12045395. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0957-9672&volume=13&issue=3&spage=261.
- ^ Ha YL, Grimm NK, Pariza MW (1987). "Anticarcinogens from fried ground beef: heat-altered derivatives of linoleic acid". Carcinogenesis 8 (12): 1881–7. PMID 3119246.
- ^ "CLA: Conjugated Linoleic Acid - Google Book Search". http://books.google.com/books?hl=en&id=su_k_WkP0KgC&dq=Conjugated+linoleic+acid+Williams&printsec=frontcover&source=web&ots=W-aRO2HX46&sig=20WMkVLUeV-AXmYtcIZwEgF14GU&sa=X&oi=book_result&resnum=10&ct=result#PPP1,M1.
- ^ a b "Fatty Acid Profiles of Liver, Adipose Tissue, Speen, and Heart of Mice Fed Diets Containing T10, C-12-, and C9, T11-Conjugated Linoleic Adic". http://www.ars.usda.gov/research/publications/publications.htm?seq_no_115=179685.
- ^ II International Congress on CLA from Experimental Models to Human Application
- ^ Kuhnt K, Kraft J, Moeckel P, Jahreis G (April 2006). "Trans-11-18 : 1 is effectively Delta9-desaturated compared with trans-12-18 : 1 in humans". Br J Nutr. 95 (4): 752–761. doi:. PMID 16571155.
- ^ Banni S, Angioni E, Murru E, Carta G, Melis M, Bauman D, Dong Y, Ip C (2001). "Vaccenic acid feeding increases tissue levels of conjugated linoleic acid and suppresses development of premalignant lesions in rat mammary gland". Nutr Cancer 41 (1-2): 91–7. doi:. PMID 12094634.
- ^ Belury, M.A. (October 2002). "Inhibition of carcinogenesis by conjugated linoleic acid: Potential mechanisms of action". Journal of Nutrition 132 (10): 2995–2998. PMID 12368384.
- ^ a b Amarù DL, Field CJ. (2009), "Conjugated Linoleic Acid Decreases MCF-7 Human Breast Cancer Cell Growth and Insulin-Like Growth Factor-1 Receptor Levels.", Lipids 26, PMID 19266226
- ^ Lee Y, Thompson JT, de Lera AR, Vanden Heuvel JP. (2008), "Isomer-specific effects of conjugated linoleic acid on gene expression in RAW 264.7.", J Nutr Biochem 26, PMID 18993052
- ^ Coakley M, Banni S, Johnson MC, Mills S, Devery R, Fitzgerald G, Paul Ross R, Stanton C. (2009), "Inhibitory Effect of Conjugated alpha-Linolenic Acid from Bifidobacteria of Intestinal Origin on SW480 Cancer Cells.", Lipids 44, PMID 19048324
- ^ Ip C, Scimeca JA, Thompson HJ. (1994), "Conjugated linoleic acid. A powerful anticarcinogen from animal fat sources.", Cancer 233 (3): 1050-4, PMID 8039138
- ^ Kritchevsky D. (May 2000), "Antimutagenic and some other effects of conjugated linoleic acid.", Br J Nutr. 83 (5): 459-65, PMID 10953669
- ^ Pariza MW, Park Y, Cook ME. (Jul 2001), "The biologically active isomers of conjugated linoleic acid.", Prog Lipid Res. 40 (4): 283-98, PMID 11412893
- ^ Bhattacharya A, Banu J, Rahman M, Causey J, Fernandes G. (Dec 2006), "Biological effects of conjugated linoleic acids in health and disease.", J Nutr Biochem. 17 (12): 789-810, PMID 16650752
- ^ Donnelly C, Olsen AM, Lewis LD, Eisenberg BL, Eastman A, Kinlaw WB. (2009), "Conjugated linoleic acid (CLA) inhibits expression of the Spot 14 (THRSP) and fatty acid synthase genes and impairs the growth of human breast cancer and liposarcoma cells.", Nutr Cancer. 61 (1): 114-22, PMID 19116881
- ^ Islam MA, Kim YS, Jang WJ, Lee SM, Kim HG, Kim SY, Kim JO, Ha YL. (2008), "A mixture of trans, trans conjugated linoleic acid induces apoptosis in MCF-7 human breast cancer cells with reciprocal expression of Bax and Bcl-2.", J Agric Food Chem (Korea) 56, PMID 18570428
- ^ Meng X, Shoemaker SF, McGee SO, Ip MM. (2008), "t10,c12-Conjugated linoleic acid stimulates mammary tumor progression in Her2/ErbB2 mice through activation of both proliferative and survival pathways.", Carcinogenesis (NY, USA) 29, PMID 18339686
- ^ Kim YS, Cerbo RM, Hah CK, Bahn KN, Kim JO, Ha YL. (2008), "Growth inhibition of osteosarcoma cell MG-63 by a mixture of trans,trans conjugated linoleic acid isomers: possible mechanistic actions.", J Food Sci. (Korea) 73, PMID 18211379
- ^ Kelley NS, Hubbard NE, Erickson KL. (2007), "Conjugated linoleic acid isomers and cancer.", J Nutr (UC Davis, Ca, USA) 137, PMID 18029471
- ^ Fite A, Goua M, Wahle KW, Schofield AC, Hutcheon AW, Heys SD. (2007), "Potentiation of the anti-tumour effect of docetaxel by conjugated linoleic acids (CLAs) in breast cancer cells in vitro.", Prostaglandins Leukot Essent Fatty Acids. (Scotland, UK) 77, PMID 17900885
- ^ Ou L, Ip C, Lisafeld B, Ip MM. (2007), "Conjugated linoleic acid induces apoptosis of murine mammary tumor cells via Bcl-2 loss.", Biochem Biophys Res Commun. (NY, USA) 356, PMID 17400188
- ^ Wang LS, Huang YW, Sugimoto Y (2006), "Conjugated linoleic acid (CLA) up-regulates the estrogen-regulated cancer suppressor gene, protein tyrosine phosphatase {gamma} (PTP{gamma}), in human breast cells.", Anticancer Res 26: 27–34, PMID 16475675
- ^ Lukas Rist, Andre Mueller, Christiane Barthel, Bianca Snijders, Margje Jansen, A. Paula Simoes-Wust, Machteld Huber, Ischa Kummeling, Ursula von Mandach, Hans Steinhart, and Carel Thijs. (June 2007). "Influence of organic diet on the amount of conjugated linoleic acids in breast milk". British Journal of Nutrition.
- ^ Thom E, Wadstein J, Gudmundsen O. (Sep-Oct 2001). "Conjugated linoleic acid reduces body fat in healthy exercising humans". The Journal of International Medical Research 29 (5): 392–396. PMID 11725826.
- ^ Blankson H, Stakkestad JA, Fagertun H, Thom E, Wadstein J, Gudmundsen O. (December 2000). "Conjugated linoleic acid reduces body fat mass in overweight and obese humans". Journal of Nutrition 130 (12): 2943–2948. PMID 11110851. http://jn.nutrition.org/cgi/content/full/130/12/2943. Retrieved on 2006-05-27.
- ^ ref name="PMID: 12923219" Larsen TM, Toubro S, Astrup A (December 2003). "Efficacy and safety of dietary supplements containing CLA for the treatment of obesity: evidence from animal and human studies". J. Lipid Res. 44 (12): 2234–41. doi:. PMID 12923219.
- ^ Risérus U, Basu S, Jovinge S, Fredrikson GN, Arnlöv J, Vessby B (2002). "Supplementation with conjugated linoleic acid causes isomer-dependent oxidative stress and elevated C-reactive protein: a potential link to fatty acid-induced insulin resistance". Circulation 106 (15): 1925–9. doi:. PMID 12370214.
- ^ Risérus U, Smedman A, Basu S, Vessby B (2003). "CLA and body weight regulation in humans". Lipids 38 (2): 133–7. doi:. PMID 12733745.
- ^ "Conjugated linoleic acid, unlike other unsaturated fatty acids, strongly induces glutathione synthesis without any lipoperoxidation". British Journal of Nutrition. http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=932332. Retrieved on 2008-09-30.
- ^ Ulf Risérus, MMed; Samar Basu, PhD; Stefan Jovinge, MD, PhD; Gunilla Nordin Fredrikson, PhD; Johan Ärnlöv, MD; Bengt Vessby, MD, PhD (September 2002). "Supplementation With Conjugated Linoleic Acid Causes Isomer-Dependent Oxidative Stress and Elevated C-Reactive Protein". American Heart Association Journals 106: 1925. doi:. 01.CIR.0000033589.15413.48v1. PMID 12370214. http://intl-circ.ahajournals.org/cgi/content/short/106/15/1925. Retrieved on 2007-02-19.
- ^ "A natural quick fix - What Doctors Don't Tell You". HealthWorld Online,. 2. http://www.healthy.net/scr/Article.asp?Id=3451&xcntr=2. Retrieved on 2007-11-05.
- ^ "The role of conjugated linoleic acid in reducing body fat and preventing holiday weight gain". International Journal of Obesity. http://www.nature.com/ijo/journal/v31/n3/abs/0803437a.html. Retrieved on 2008-09-30.
- ^ "The effect of 6 months supplementation with conjugated linoleic acid on insulin resistance in overweight and obese". International Journal of Obesity. http://www.nature.com/ijo/journal/v31/n7/abs/0803482a.html. Retrieved on 2008-09-30.
- ^ "Conjugated linoleic acid lowers hypercholesterolemia but increases the risk for biliary lithiasis". Nutr Hosp.. http://lib.bioinfo.pl/pmid:15989070. Retrieved on 2008-09-30.
- ^ Commonwealth Scientific and Industrial Research Organisation (CSIRO) (2004-04-23). Kangaroo meat - health secret revealed. Press release. http://www.csiro.au/files/mediarelease/mr2004/kangaroofat.htm. Retrieved on 2007-01-23.
- ^ T. R. Dhiman, L. D. Satter, M. W. Pariza, M. P. Galli, K. Albright, and M. X. Tolosa (May 2000). "Conjugated Linoleic Acid (CLA) Content of Milk from Cows Offered Diets Rich in Linoleic and Linolenic Acid". Journal of Dairy Science 83 (5): 1016–1027. PMID 10821577. http://jds.fass.org/cgi/content/abstract/83/5/1016. Retrieved on 2006-05-27.
- ^ T. R. Dhiman (2001). "Role of diet on conjugated linoleic acid content of milk and meat" (PDF). Journal of Animal Science 79. http://www.adsa.org/jointabs/iaafs108.pdf. Retrieved on 2007-03-09.
- ^ Lin Yang, Ying Cao, Zhen-Yu Chen (2004). "Stability of conjugated linoleic acid isomers in egg yolk lipids during frying". Food Chemistry (Elsevier) 86: 531–535. doi:.
- ^ Chen, S. (2006), "Anti-aromatase activity of phytochemicals in white button mushrooms (Agaricus bisporus).", Cancer Res. 66 (24): 12026-12034, PMID 17178902
[edit] General references
- Tokuşoğlu Ö. (2008). Conjugated linoleic acid (CLA) cis 9, trans 11 and trans 10, cis 12 isomer detection in crude and refined corn oils by capillary GC. Grasas y Aceites (Spain). Vol.59(2) Abril-Junio 2008, 146-151.
- Tokuşoğlu Ö., Durucasu İ., Akalın A.S., Serin E., Akşit S. (2007). Fatty Acid (FA) and Conjugated Linoleic Acid (CLA) Profiles of Infant Formulas Through Direct Transesterification of Acyl Lipids. Italian J Food Sci. No:4 Vol.19, 477-484.
- Akalın A.S., Tokuşoğlu Ö., Gonc S., Aycan Ş. (2007). Occurrence of conjugated linoleic acid in probiotic yoghurts supplemented with fructooligosaccharide. International Dairy Journal. Vol 17/9, 1089-1095.
- Schmid A., Collomb M., Sieber R., Bee G. Conjugated linoleic acid in meat and meat products: A review // Meat Science. – 2006. – 73. – P. 29–41.
- Jenkins T. C., McGuire M. A. Major advances in nutrition: impact on milk composition // J. Dairy Sci. – 2006. – 89 (4) – Р. 1302–1310.
- Akalın A.S., Tokuşoğlu Ö., Gönç S., Ökten S.(2005). “Detection of Biologically Active Isomers of Conjugated Linoleic Acid in "Kaymak". Grasas Aceties 56(4), 298-302.
- Al Sarakbi W, Salhab M, Mokbel K. Dairy products and breast cancer risk: a review of the literature. Int J Fertil Women's Med. 2005 Nov-Dec;50(6):244-9. Review.
- Bhattacharya A, Banu J, Rahman M, Causey J, Fernandes G. Biological effects of conjugated linoleic acids in health and disease. J Nutr Biochem. 2006 Dec;17(12):789-810. Epub 2006 May 2. Review.
- Ip MM, Masso-Welch PA, Ip C. Prevention of mammary cancer with conjugated linoleic acid: role of the stroma and the epithelium. J Mammary Gland Biol Neoplasia. 2003 Jan;8(1):103-18. Review.
- Kritchevsky D. Antimutagenic and some other effects of conjugated linoleic acid // British Journal of Nutrition. – 2000. – 83, N 5. – P. 459-465.
- Larsson S. C., Bergkvist L., Wolk A. High-fat dairy food and conjugated linoleic acid intakes in relation to colorectal cancer incidence in the Swedish Mammography Cohort // American Journal of Clinical Nutrition. – 2005. – 82, N 4. – P. 894-900.
- Lee KW, Lee HJ, Cho HY, Kim YJ. Role of the conjugated linoleic acid in the prevention of cancer. Crit Rev Food Sci Nutr. 2005;45(2):135-44. Review.
- Maynard L. J., Franklin S. T. Functional foods as a value-added strategy: the commercial potential of "cancer-fighting" dairy products // Review of Agricultural Economics. – 2003. – 25, N 2. – P. 316-331.
- Miller Á., Stanton C., Murphy J., Devery R. Conjugated linoleic acid (CLA)-enriched milk fat inhibits growth and modulates CLA-responsive biomarkers in MCF-7 and SW480 human cancer cell lines // British Journal of Nutrition. – 2003. – 90, N 5. – P. 877-885.
- Pariza MW, Park Y, Cook ME. Conjugated linoleic acid and the control of cancer and obesity. Toxicol Sci. 1999 Dec;52 (2 Suppl):107-10. Review.
- Tanaka K. Occurrence of conjugated linoleic acid in ruminant products and its physiological functions // Animal Science Journal. – 2005. – 76, N 4. – P. 291-303.
- Voorrips L. E., Brants H. A. M., Kardinaal A. F. M., Hiddink G. J., Brandt P. A., van den Goldbohm R. A. Intake of conjugated linoleic acid, fat, and other fatty acids in relation to postmenopausal breast cancer: the Netherlands cohort study on diet and cancer // American Journal of Clinical Nutrition. – 2002. – 76, N 4. – P. 873-882.
- Belury M. A. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action // Annu. Rev. Nutr. – 2002. – 22. – P. 505–531.
- Bauman D. E., Corl B. A., Baumgard L. H., Griinari J. M. Conjugated linoleic acid (CLA) and the dairy cow // In Recent Advances in Animal Nutrition / Ed. P. C. Garnsworthy, J. Wiseman; Nottingham Univ. Press. – Nottingham, UK, 2001 – P. 221–250.
- Harefoot C. G., Hazlewood G. P. Lipid metabolism in the rumen / In: Hobson P. N., Stewart C. S. (Eds.), The Rumen Microbial Ecosystem, second ed. Blackie Academic, London, 1999. – P. 382–426.

