Gold
From Wikipedia, the free encyclopedia
|
||||||||||||||||||||||||||||||||||||||||
| General | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | gold, Au, 79 | |||||||||||||||||||||||||||||||||||||||
| Element category | transition metals | |||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 11, 6, d | |||||||||||||||||||||||||||||||||||||||
| Appearance | metallic yellow |
|||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 196.966569(4) g·mol−1 | |||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d10 6s1 | |||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 1 | |||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||
| Phase | solid | |||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 19.3 g·cm−3 | |||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 17.31 g·cm−3 | |||||||||||||||||||||||||||||||||||||||
| Melting point | 1337.33 K (1064.18 °C, 1947.52 °F) |
|||||||||||||||||||||||||||||||||||||||
| Boiling point | 3129 K (2856 °C, 5173 °F) |
|||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 12.55 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 324 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 25.418 J·mol−1·K−1 | |||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||
| Crystal structure | cubic face centered | |||||||||||||||||||||||||||||||||||||||
| Oxidation states | −1, 1, 2, 3, 4, 5 (amphoteric oxide) |
|||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.54 (Pauling scale) | |||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 890.1 kJ/mol | |||||||||||||||||||||||||||||||||||||||
| 2nd: 1980 kJ/mol | ||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 135 pm | |||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 174 pm | |||||||||||||||||||||||||||||||||||||||
| Covalent radius | 144 pm | |||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 166 pm | |||||||||||||||||||||||||||||||||||||||
| Miscellaneous | ||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic | |||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 22.14 n Ω·m | |||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 318 W·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 14.2 µm·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | (r.t.) (hard-drawn) 2030 m·s−1 |
|||||||||||||||||||||||||||||||||||||||
| Young's modulus | 78 GPa | |||||||||||||||||||||||||||||||||||||||
| Tensile strain | 0.00157 | |||||||||||||||||||||||||||||||||||||||
| Shear modulus | 27 GPa | |||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 180 GPa | |||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.44 | |||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 2.5 | |||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 216 MPa | |||||||||||||||||||||||||||||||||||||||
| Brinell hardness | ? 2450 MPa | |||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-57-5 | |||||||||||||||||||||||||||||||||||||||
| Most-stable isotopes | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||
Gold (pronounced /ˈɡoʊld/) is a chemical element with the symbol Au (Latin: aurum) and atomic number 79. It is a highly sought-after precious metal in jewelry, in sculpture, and for ornamentation since the beginning of recorded history. The metal occurs as nuggets or grains in rocks, in veins and in alluvial deposits. Gold is dense, soft, shiny and the most malleable and ductile pure metal known. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without rusting in air or water. It is one of the coinage metals and formed the basis for the gold standard used before the collapse of the Bretton Woods system in 1971.
Modern industrial uses include dentistry and electronics, where gold has traditionally found use because of its good resistance to oxidative corrosion. Chemically, gold is a transition metal and can form trivalent and univalent cations upon solvation. At STP it is attacked by aqua regia (a mixture of acids), forming chloroauric acid and by alkaline solutions of cyanide but not by single acids such as hydrochloric, nitric or sulfuric acids. Gold dissolves in mercury, forming amalgam alloys, but does not react with it. Gold is insoluble in nitric acid, which will dissolve silver and base metals, and is the basis of the gold refining technique known as "inquartation and parting". Nitric acid has long been used to confirm the presence of gold in items, and this is the origin of the colloquial term "acid test", referring to a gold standard test for genuine value.
Contents |
[edit] Characteristics
Gold is the most malleable and ductile of all metals; a single gram can be beaten into a sheet of one square meter, or an ounce into 300 square feet. Gold leaf can be beaten thin enough to become translucent. The transmitted light appears greenish blue, because gold strongly reflects yellow and red.
Gold readily creates alloys with many other metals. These alloys can be produced to modify the hardness and other metallurgical properties, to control melting point or to create exotic colors (see below). Gold is a good conductor of heat and electricity and reflects infra red radiation strongly. Chemically, it is unaffected by air, moisture and most corrosive reagents, and is therefore well-suited for use in coins and jewelry and as a protective coating on other, more reactive, metals. However, it is not chemically inert. Free halogens will react with gold, and aqua regia dissolves it via formation of chlorine gas which attacks gold to form the chloraurate ion. Gold also dissolves in alkaline solutions of potassium cyanide and in mercury, forming a gold-mercury amalgam.
Common oxidation states of gold include +1 (gold(I) or aurous compounds) and +3 (gold(III) or auric compounds). Gold ions in solution are readily reduced and precipitated out as gold metal by adding any other metal as the reducing agent. The added metal is oxidized and dissolves allowing the gold to be displaced from solution and be recovered as a solid precipitate.
High quality pure metallic gold is tasteless; in keeping with its resistance to corrosion (it is metal ions which confer taste to metals).
In addition, gold is very dense, a cubic meter weighing 19300 kg. By comparison, the density of lead is 11340 kg/m³, and that of the densest element, osmium, is 22610 kg/m³.
[edit] Color of gold
The color of pure gold is metallic yellow. Gold, caesium and copper are the only metallic elements with a natural color other than gray or white. The usual gray color of metals depends on their "electron sea" that is capable of absorbing and re-emitting photons over a wide range of frequencies. Gold reacts differently, depending on subtle relativistic effects that affect the orbitals around gold atoms.[1][2]
Common colored gold alloys such as rose gold can be created by the addition of various amounts of copper and silver, as indicated in the diagram below. Alloys containing palladium or nickel are also important in commercial jewelry as these produce white gold alloys. Less commonly, addition of manganese, aluminium, iron, indium and other elements can produce more unusual colors of gold for various applications.[3]
[edit] Applications
[edit] As the metal
[edit] Medium of monetary exchange
In various countries, gold was used as a standard for monetary exchange, but this practice has been abandonded with the rise of fiat currency. The last country to back their money with gold was Switzerland, which backed 40% of its value until it joined the International Monetary Fund in 1999. [4] Pure gold is too soft for ordinary use and is typically hardened by alloying with copper or other base metals. The gold content of gold alloys is measured in carats (k), pure gold being designated as 24k.

Gold coins intended for circulation from 1526 into the 1930s were typically a standard 22k alloy called crown gold, for hardness. Modern collector/investment bullion coins (which do not require good mechanical wear properties) are typically 24k, although the American Gold Eagle, the British gold sovereign and the South African Krugerrand continue to be made at 22k, on historical tradition. The special issue Canadian Gold Maple Leaf coin contains the highest purity gold of any bullion coin, at 99.999% (.99999 fine). The popular issue Canadian Gold Maple Leaf coin has a purity of 99.99%. Several other 99.99% pure gold coins are currently available, including Australia's Gold Kangaroos (first appearing in 1986 as the Australian Gold Nugget, with the kangaroo theme appearing in 1989), the several coins of the Australian Lunar Calendar series, and the Austrian Philharmonic. In 2006, the U.S. Mint began production of the American Buffalo gold bullion coin also at 99.99% purity.
Gold was used as a medium of monetary exchange throughout history together or instead of other minerals, like silver, salt, and copper. After World War II gold was replaced by a system of convertible currency following the Bretton Woods system. Many holders of gold in storage (as bullion coin or bullion) hold it as a hedge against inflation or other economic disruptions. The ISO currency code of gold bullion is XAU.
| The examples and perspective in this section deal primarily with Anglosphere and do not represent a worldwide view of the subject. Please improve this article or discuss the issue on the talk page. |
[edit] Jewelry
Because of the softness of pure (24k) gold, it is usually alloyed with base metals for use in jewelry, altering its hardness and ductility, melting point, color and other properties. Alloys with lower caratage, typically 22k, 18k, 14k or 10k, contain higher percentages of copper, or other base metals or silver or palladium in the alloy. Copper is the most commonly used base metal, yielding a redder color. Eighteen carat gold containing 25% copper is found in antique and Russian jewellery and has a distinct, though not dominant, copper cast, creating rose gold. Fourteen carat gold-copper alloy is nearly identical in color to certain bronze alloys, and both may be used to produce police, as well as other, badges. Blue gold can be made by alloying with iron and purple gold can be made by alloying with aluminium, although rarely done except in specialized jewelry. Blue gold is more brittle and therefore more difficult to work with when making jewelry. Fourteen and eighteen carat gold alloys with silver alone appear greenish-yellow and are referred to as green gold. White gold alloys can be made with palladium or nickel. White 18 carat gold containing 17.3% nickel, 5.5% zinc and 2.2% copper is silver in appearance. Nickel is toxic, however, and its release from nickel white gold is controlled by legislation in Europe. Alternative white gold alloys are available based on palladium, silver and other white metals (World Gold Council), but the palladium alloys are more expensive than those using nickel. High-carat white gold alloys are far more resistant to corrosion than are either pure silver or sterling silver. The Japanese craft of Mokume-gane exploits the color contrasts between laminated colored gold alloys to produce decorative wood-grain effects.
[edit] Medicine
| This article is in a list format that may be better presented using prose. You can help by converting this section to prose, if appropriate. Editing help is available. (January 2009) |
- In medieval times, gold was often seen as beneficial for the health, in the belief that something that rare and beautiful could not be anything but healthy.[citation needed] Even some modern esotericists and forms of alternative medicine assign metallic gold a healing power.[citation needed] Some gold salts do have anti-inflammatory properties and are used as pharmaceuticals in the treatment of arthritis and other similar conditions. However, only salts and radioisotopes of gold are of pharmacological value, as elemental (metallic) gold is inert to all chemicals it encounters inside the body.[citation needed]
- In modern times injectable gold has been proven to help to reduce the pain and swelling of rheumatoid arthritis.[5]
- Dentistry. Gold alloys are used in restorative dentistry, especially in tooth restorations, such as crowns and permanent bridges. The gold alloys' slight malleability facilitates the creation of a superior molar mating surface with other teeth and produces results that are generally more satisfactory than those produced by the creation of porcelain crowns. The use of gold crowns in more prominent teeth such as incisors is favored in some cultures and discouraged in others.
- Colloidal gold (colloidal sols of gold nanoparticles) in water are intensely red-colored, and can be made with tightly-controlled particle sizes up to a few tens of nm across by reduction of gold chloride with citrate or ascorbate ions. Colloidal gold is used in research applications in medicine, biology and materials science. The technique of immunogold labeling exploits the ability of the gold particles to adsorb protein molecules onto their surfaces. Colloidal gold particles coated with specific antibodies can be used as probes for the presence and position of antigens on the surfaces of cells (Faulk and Taylor 1979). In ultrathin sections of tissues viewed by electron microscopy, the immunogold labels appear as extremely dense round spots at the position of the antigen (Roth et al. 1980). Colloidal gold is also the form of gold used as gold paint on ceramics prior to firing.
- Gold, or alloys of gold and palladium, are applied as conductive coating to biological specimens and other non-conducting materials such as plastics and glass to be viewed in a scanning electron microscope. The coating, which is usually applied by sputtering with an argon plasma, has a triple role in this application. Gold's very high electrical conductivity drains electrical charge to earth, and its very high density provides stopping power for electrons in the SEM's electron beam, helping to limit the depth to which the electron beam penetrates the specimen. This improves definition of the position and topography of the specimen surface and increases the spatial resolution of the image. Gold also produces a high output of secondary electrons when irradiated by an electron beam, and these low-energy electrons are the most commonly-used signal source used in the scanning electron microscope.
- The isotope gold-198, (half-life: 2.7 days) is used in some cancer treatments and for treating other diseases.[6]
[edit] Food and drink
- Gold can be used in food and has the E Number 175.
- Gold leaf, flake or dust is used on and in some gourmet foodstuffs, notably sweets and drinks as decorative ingredient.[7] Gold flake was used by the nobility in Medieval Europe as a decoration in foodstuffs and drinks, in the form of leaf, flakes or dust, either to demonstrate the host's wealth or in the belief that something that valuable and rare must be beneficial for one's health.
- Goldwasser (English: Goldwater) is a traditional herbal liqueur produced in Gdańsk, Poland, and Schwabach, Germany, and contains flakes of gold leaf. There are also some expensive (~$1000) cocktails which contain flakes of gold leaf[citation needed]. However, since metallic gold is inert to all body chemistry, it adds no taste nor has it any other nutritional effect and leaves the body unaltered.[citation needed]
[edit] Industry
- Gold solder is used for joining the components of gold jewelry by high-temperature hard soldering or brazing. If the work is to be of hallmarking quality, gold solder must match the carat weight of the work, and alloy formulas are manufactured in most industry-standard carat weights to color match yellow and white gold. Gold solder is usually made in at least three melting-point ranges referred to as Easy, Medium and Hard. By using the hard, high-melting point solder first, followed by solders with progressively lower melting points, goldsmiths can assemble complex items with several separate soldered joints.
- Gold can be made into thread and used in embroidery.
- Gold is ductile and malleable, meaning it can be drawn into very thin wire and can be beaten into very thin sheets known as gold leaf.
- Gold produces a deep, intense red color when used as a coloring agent in cranberry glass.
- In photography, gold toners are used to shift the color of silver bromide black and white prints towards brown or blue tones, or to increase their stability. Used on sepia-toned prints, gold toners produce red tones. Kodak published formulas for several types of gold toners, which use gold as the chloride (Kodak, 2006).
- As gold is a good reflector of electromagnetic radiation such as infrared and visible light as well as radio waves, it is used for the protective coatings on many artificial satellites, in infrared protective faceplates in thermal protection suits and astronauts' helmets and in electronic warfare planes like the EA-6B Prowler.
- Gold is used as the reflective layer on some high-end CDs.
- Automobiles may use gold for heat insulation. McLaren uses gold foil in the engine compartment of its F1 model.[8]
[edit] Electronics
- The concentration of free electrons in gold metal is 5.90×1022 cm-3. Gold is highly conductive to electricity, and has been used for electrical wiring in some high energy applications (silver is even more conductive per volume, but gold has the advantage of corrosion resistance). For example, gold electrical wires were used during some of the Manhattan Project's atomic experiments, but large high current silver wires were used in the calutron isotope separator magnets in the project.
- Though gold is attacked by free chlorine, its good conductivity and general resistance to oxidation and corrosion in other environments (including resistance to non-chlorinated acids) has led to its widespread industrial use in the electronic era as a thin layer coating electrical connectors of all kinds, thereby ensuring good connection. For example, gold is used in the connectors of the more expensive electronics cables, such as audio, video and USB cables. The benefit of using gold over other connector metals such as tin in these applications is highly debated. Gold connectors are often criticized by audio-visual experts as unnecessary for most consumers and seen as simply a marketing ploy. However, the use of gold in other applications in electronic sliding contacts in highly humid or corrosive atmospheres, and in use for contacts with a very high failure cost (certain computers, communications equipment, spacecraft, jet aircraft engines) remains very common, and is unlikely to be replaced in the near future by any other metal.
- Besides sliding electrical contacts, gold is also used in electrical contacts because of its resistance to corrosion, electrical conductivity, ductility and lack of toxicity.[9] Switch contacts are generally subjected to more intense corrosion stress than are sliding contacts.
[edit] Other
- Many competitions, and honors, such as the Olympics and the Nobel Prize, award a gold medal to the winner.
[edit] As gold chemical compounds
Gold is attacked by and dissolves in alkaline solutions of potassium or sodium cyanide, and gold cyanide is the electrolyte used in commercial electroplating of gold onto base metals and electroforming. Gold chloride (chloroauric acid) solutions are used to make colloidal gold by reduction with citrate or ascorbate ions. Gold chloride and gold oxide are used to make highly-valued cranberry or red-colored glass, which, like colloidal gold sols, contains evenly-sized spherical gold nanoparticles.[citation needed]
[edit] History


Gold has been known and highly valued since prehistoric times. It may have been the first metal used by humans and was valued for ornamentation and rituals. Egyptian hieroglyphs from as early as 2600 BC describe gold, which king Tushratta of the Mitanni claimed was "more plentiful than dirt" in Egypt.[10] Egypt and especially Nubia had the resources to make them major gold-producing areas for much of history. The earliest known map is known as the Turin papyrus and shows the plan of a gold mine in Nubia together with indications of the local geology. The primitive working methods are described by Strabo and included fire-setting. Large mines also occurred across the Red Sea in what is now Saudi Arabia.



The legend of the golden fleece may refer to the use of fleeces to trap gold dust from placer deposits in the ancient world. Gold is mentioned frequently in the Old Testament, starting with Genesis 2:11 (at Havilah) and is included with the gifts of the magi in the first chapters of Matthew New Testament. The Book of Revelation 21:21 describes the city of New Jerusalem as having streets "made of pure gold, clear as crystal". The south-east corner of the Black Sea was famed for its gold. Exploitation is said to date from the time of Midas, and this gold was important in the establishment of what is probably the world's earliest coinage in Lydia between 643 and 630 BC.
From 6th or 5th century BCE, Chu (state) circulated Ying Yuan, one kind of square gold coin.
The Romans developed new methods for extracting gold on a large scale using hydraulic mining methods, especially in Spain from 25 BC onwards and in Romania from 150 AD onwards. One of their largest mines was at Las Medulas in León (Spain), where seven long aqueducts enabled them to sluice most of a large alluvial deposit. The mines at Roşia Montană in Transylvania were also very large, and until very recently, still mined by opencast methods. They also exploited smaller deposits in Britain, such as placer and hard-rock deposits at Dolaucothi. The various methods they used are well described by Pliny the Elder in his encyclopedia Naturalis Historia written towards the end of the first century AD.
The Mali Empire in Africa was famed throughout the old world for its large amounts of gold. Mansa Musa, ruler of the empire (1312–1337) became famous throughout the old world for his great hajj to Mecca in 1324. When he passed through Cairo in July of 1324, he was reportedly accompanied by a camel train that included thousands of people and nearly a hundred camels. He gave away so much gold that it depressed the price in Egypt for over a decade.[11] A contemporary Arab historian remarked:
| “ | Gold was at a high price in Egypt until they came in that year. The mithqal did not go below 25 dirhams and was generally above, but from that time its value fell and it cheapened in price and has remained cheap till now. The mithqal does not exceed 22 dirhams or less. This has been the state of affairs for about twelve years until this day by reason of the large amount of gold which they brought into Egypt and spent there [...] | ” |
The European exploration of the Americas was fueled in no small part by reports of the gold ornaments displayed in great profusion by Native American peoples, especially in Central America, Peru, Ecuador and Colombia.
Although the price of some platinum group metals can be much higher, gold has long been considered the most desirable of precious metals, and its value has been used as the standard for many currencies (known as the gold standard) in history. Gold has been used as a symbol for purity, value, royalty, and particularly roles that combine these properties. Gold as a sign of wealth and prestige was made fun of by Thomas More in his treatise Utopia. On that imaginary island, gold is so abundant that it is used to make chains for slaves, tableware and lavatory-seats. When ambassadors from other countries arrive, dressed in ostentatious gold jewels and badges, the Utopians mistake them for menial servants, paying homage instead to the most modestly-dressed of their party.
There is an age-old tradition of biting gold in order to test its authenticity. Although this is certainly not a professional way of examining gold, the bite test should score the gold because gold is a soft metal, as indicated by its score on the Mohs' scale of mineral hardness. The purer the gold the easier it should be to mark it. Painted lead can cheat this test because lead is softer than gold (and may invite a small risk of lead poisoning if sufficient lead is absorbed by the biting).
Gold in antiquity was relatively easy to obtain geologically; however, 75% of all gold ever produced has been extracted since 1910.[13] It has been estimated that all the gold in the world that has ever been refined would form a single cube 20 m (66 ft) on a side (equivalent to 8000 m³).[13]
One main goal of the alchemists was to produce gold from other substances, such as lead — presumably by the interaction with a mythical substance called the philosopher's stone. Although they never succeeded in this attempt, the alchemists promoted an interest in what can be done with substances, and this laid a foundation for today's chemistry. Their symbol for gold was the circle with a point at its center (☉), which was also the astrological symbol, and the ancient Chinese character, for the Sun. For modern creation of artificial gold by neutron capture, see gold synthesis.
During the 19th century, gold rushes occurred whenever large gold deposits were discovered. The first documented discovery of gold in the United States was at the Reed Gold Mine near Georgeville, North Carolina in 1803.[14] The first major gold strike in the United States occurred in a small north Georgia town called Dahlonega.[15] Further gold rushes occurred in California, Colorado, Otago, Australia, Witwatersrand, Black Hills, and Klondike.
Because of its historically high value, much of the gold mined throughout history is still in circulation in one form or another.
[edit] Occurrence
In nature, gold most often occurs in its native state (that is, as a metal), though usually alloyed with silver. Native gold contains usually eight to ten percent silver, but often much more — alloys with a silver content over 20% are called electrum. As the amount of silver increases, the color becomes whiter and the specific gravity becomes lower.
Ores bearing native gold consist of grains or microscopic particles of metallic gold embedded in rock, often in association with veins of quartz or sulfide minerals like pyrite. These are called "lode" deposits. Native gold is also found in the form of free flakes, grains or larger nuggets that have been eroded from rocks and end up in alluvial deposits (called placer deposits). Such free gold is always richer at the surface of gold-bearing veins owing to the oxidation of accompanying minerals followed by weathering, and washing of the dust into streams and rivers, where it collects and can be welded by water action to form nuggets.
Gold sometimes occurs combined with tellurium as the minerals calaverite, krennerite, nagyagite, petzite and sylvanite, and as the rare bismuthide maldonite (Au2Bi) and antimonide aurostibite (AuSb2). Gold also occurs in rare alloys with copper, lead, and mercury: the minerals auricupride (Cu3Au), novodneprite (AuPb3) and weishanite ((Au,Ag)3Hg2).
Recent research suggests that microbes can sometimes play an important role in forming gold deposits, transporting and precipitating gold to form grains and nuggets that collect in alluvial deposits.[16]
[edit] Production
Economic gold extraction can be achieved from ore grades as little as 0.5 g/1000 kg (0.5 parts per million, ppm) on average in large easily mined deposits. Typical ore grades in open-pit mines are 1–5 g/1000 kg (1–5 ppm); ore grades in underground or hard rock mines are usually at least 3 g/1000 kg (3 ppm). Because ore grades of 30 g/1000 kg (30 ppm) are usually needed before gold is visible to the naked eye, in most gold mines the gold is invisible.
Since the 1880s, South Africa has been the source for a large proportion of the world’s gold supply, with about 50% of all gold ever produced having come from South Africa. Production in 1970 accounted for 79% of the world supply, producing about 1,000 tonnes. However by 2007 production was just 272 tonnes. This sharp decline was due to the increasing difficulty of extraction, changing economic factors affecting the industry, and tightened safety auditing. In 2007 China (with 276 tonnes) overtook South Africa as the world's largest gold producer, the first time since 1905 that South Africa has not been the largest.[17]
The city of Johannesburg located in South Africa was founded as a result of the Witwatersrand Gold Rush which resulted in the discovery of some of the largest gold deposits the world has ever seen. Gold fields located within the basin in the Free State and Gauteng provinces are extensive in strike and dip requiring some of the world's deepest mines, with the Savuka and TauTona mines being currently the world's deepest gold mine at 3,777 m. The Second Boer War of 1899–1901 between the British Empire and the Afrikaner Boers was at least partly over the rights of miners and possession of the gold wealth in South Africa.
Other major producers are the United States, Australia, Russia and Peru. Mines in South Dakota and Nevada supply two-thirds of gold used in the United States. In South America, the controversial project Pascua Lama aims at exploitation of rich fields in the high mountains of Atacama Desert, at the border between Chile and Argentina. Today about one-quarter of the world gold output is estimated to originate from artisanal or small scale mining.[18]
After initial production, gold is often subsequently refined industrially by the Wohlwill process or the Miller process. Other methods of assaying and purifying smaller amounts of gold include parting and inquartation as well as cuppelation, or refining methods based on the dissolution of gold in aqua regia.
The world's oceans hold a vast amount of gold, but in very low concentrations (perhaps 1–2 parts per 10 billion, e.g. every cubic kilometer of water could contain 10 to 20 kg of gold). A number of people have claimed to be able to economically recover gold from sea water, but so far they have all been either mistaken or crooks. Reverend Prescott Jernegan ran a gold-from-seawater swindle in America in the 1890s. A British fraudster ran the same scam in England in the early 1900s.[19]
Fritz Haber (the German inventor of the Haber process) attempted commercial extraction of gold from sea water in an effort to help pay Germany's reparations following World War I. Unfortunately, his assessment of the concentration of gold in sea water was unduly high, probably due to sample contamination. The effort produced little gold and cost the German government far more than the commercial value of the gold recovered.[citation needed] No commercially viable mechanism for performing gold extraction from sea water has yet been identified. Gold synthesis is not economically viable and is unlikely to become so in the foreseeable future.
The average gold mining and extraction costs[when?] are $238 per troy ounce but these can vary widely depending on mining type and ore quality. In 2001, global mine production amounted to 2,604 tonnes, or 67% of total gold demand in that year. At the end of 2006, it was estimated that all the gold ever mined totaled 158,000 tonnes.[20] This can be represented by a cube with an edge length of just 20.2 meters.
Gold is so stable and so valuable that it is always recovered and recycled. There is no true "consumption" of gold in the economic sense; the stock of gold remains essentially constant while ownership shifts from one party to another. [21]
[edit] Price
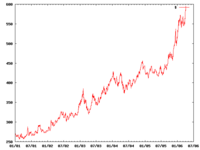
Like other precious metals, gold is measured by troy weight and by grams. When it is alloyed with other metals the term carat or karat is used to indicate the amount of gold present, with 24 karats being pure gold and lower ratings proportionally less. The purity of a gold bar can also be expressed as a decimal figure ranging from 0 to 1, known as the millesimal fineness, such as 0.995 being very pure.
The price of gold is determined on the open market, but a procedure known as the Gold Fixing in London, originating in September 1919, provides a daily benchmark figure to the industry. The afternoon fixing appeared in 1968 to fix a price when US markets are open.
Historically gold coinage was widely used as currency; When paper money was introduced, it typically was a receipt redeemable for gold coin or bullion. In an economic system known as the gold standard, a certain weight of gold was given the name of a unit of currency. For a long period, the United States government set the value of the US dollar so that one troy ounce was equal to $20.67 ($664.56/kg), but in 1934 the dollar was devalued to $35.00 per troy ounce ($1125.27/kg). By 1961 it was becoming hard to maintain this price, and a pool of US and European banks agreed to manipulate the market to prevent further currency devaluation against increased gold demand.
On March 17, 1968, economic circumstances caused the collapse of the gold pool, and a two-tiered pricing scheme was established whereby gold was still used to settle international accounts at the old $35.00 per troy ounce ($1.13/g) but the price of gold on the private market was allowed to fluctuate; this two-tiered pricing system was abandoned in 1975 when the price of gold was left to find its free-market level. Central banks still hold historical gold reserves as a store of value although the level has generally been declining. The largest gold depository in the world is that of the U.S. Federal Reserve Bank in New York, which holds about 3%[citation needed] of the gold ever mined, as does the similarly-laden U.S. Bullion Depository at Fort Knox.
In 2005 the World Gold Council estimated total global gold supply to be 3,859 tonnes and demand to be 3,754 tonnes, giving a surplus of 105 tonnes.[22]
[edit] Price records
Since 1968 the price of gold on the open market has ranged widely, from a high of $850/oz ($27,300/kg) on January 21, 1980, to a low of $252.90/oz ($8,131/kg) on June 21, 1999 (London Gold Fixing).[23] The 1980 high was not overtaken until January 3, 2008 when a new maximum of $865.35 per troy ounce was set (a.m. London Gold Fixing).[24] The current record price was set on March 17, 2008 at $1023.50/oz (am. London Gold Fixing).[24]
[edit] Long term price trends
Since April 2001 the gold price has more than tripled in value against the US dollar,[25] prompting speculation that this long secular bear market (or the Great Commodities Depression) has ended and a bull market has returned.[26] In March 2008, the gold price increased above $1000,[27] which in real terms is still well below the $850/oz. peak on January 21, 1980. Indexed for inflation, the 1980 high would equate to a price of around $2400 in 2007 US dollars.
In the last century, major economic crises (such as the Great Depression, World War II, the first and second oil crisis) lowered the Dow/Gold ratio (which is inherently inflation adjusted) substantially, in most cases to a value well below 4.[28] During these difficult times, investors tried to preserve their assets by investing in precious metals, most notably gold and silver.
[edit] Compounds
Although gold is a noble metal, it forms many and diverse compounds. The oxidation state of gold in its compound ranges from −1 to +5 but Au(I) and Au(III) dominate. Gold(I), referred to as the aurous ion, is the most common oxidation state with “soft” ligands such as thioethers, thiolates, and tertiary phosphines. Au(I) compounds are typically linear. A good example is Au(CN)2−, which is the soluble form of gold encountered in mining. Curiously, aurous complexes of water are rare. The binary gold halides, such as AuCl, form zig-zag polymeric chains, again featuring linear coordination at Au. Most drugs based on gold are Au(I) derivatives.[29]
Gold(III) (“auric”) is a common oxidation state and is illustrated by gold(III) chloride, AuCl3. Its derivative is chloroauric acid, HAuCl4, which forms when Au dissolves in aqua regia. Au(III) complexes, like other d8 compounds, are typically square planar.
[edit] Less common oxidation states: Au(-I), Au(II), and Au(V)
Compounds containing the Au− anion are called aurides. Caesium auride, CsAu which crystallizes in the caesium chloride motif.[30] Other aurides include those of Rb+, K+, and tetramethylammonium (CH3)4N+.[31] Gold(II) compounds are usually diamagnetic with Au-Au bonds such as [Au(CH2)2P(C6H5)2]2Cl2. A noteworthy, legitimate Au(II) complex is the tetraxenonogold(II) cation, which contains xenon as a ligand, [AuXe4](Sb2F11)2.[32] Gold pentafluoride is the sole example of Au(V), the highest verified oxidation state.[33]
Some gold compounds exhibit aurophilic bonding, which describes the tendency of gold ions to interact at distances that are too long to be a conventional Au-Au bond but shorter that van der Waals bonding. The interaction is estimated to be comparable in strength to that of a hydrogen bond.
[edit] Mixed valence compounds
Well-defined cluster compounds are numerous.[31] In such cases, gold has a fractional oxidation state. A representative example is the octahedral species {Au(P(C6H5)3)}62+. Gold chalcogenides, e.g. "AuS" feature equal amounts of Au(I) and Au(III).
[edit] Isotopes
Gold has only one stable isotope, 197Au, which is also its only naturally-occurring isotope. 36 radioisotopes have been synthesized ranging in atomic mass from 169 to 205. The most stable of these is 195Au with a half-life of 186.1 days. 195Au is also the only isotope to decay by electron capture. The least stable is 171Au, which decays by proton emission with a half-life of 30 µs. Most of gold's radioisotopes with atomic masses below 197 decay by some combination of proton emission, α decay, and β+ decay. The exceptions are 195Au, which decays by electron capture, and 196Au, which has a minor β- decay path. All of gold's radioisotopes with atomic masses above 197 decay by β- decay.[34]
At least 32 nuclear isomers have also been characterized, ranging in atomic mass from 170 to 200. Within that range, only 178Au, 180Au, 181Au, 182Au, and 188Au do not have isomers. Gold's most stable isomer is 198m2Au with a half-life of 2.27 days. Gold's least stable isomer is 177m2Au with a half-life of only 7 ns. 184m1Au has three decay paths: β+ decay, isomeric transition, and alpha decay. No other isomer or isotope of gold has three decay paths.[34]
[edit] Symbolism

Gold has been associated with the extremities of utmost evil and great sanctity throughout history. In the Book of Exodus, the Golden Calf is a symbol of idolatry and rebellion against God. In popular culture, the golden pocket watch and its fastening golden chain were the characteristic accessories of the capitalists, the rich and the industrial tycoons. Credit card companies associate their product with wealth by naming and coloring their top-of-the-range cards “gold” although, in an attempt to out-do each other, platinum has now overtaken gold.
In the Book of Genesis, Abraham was said to be rich in gold and silver, and Moses was instructed to cover the Mercy Seat of the Ark of the Covenant with pure gold. Eminent orators such as John Chrysostom were said to have a “mouth of gold with a silver tongue.” Gold is associated with notable anniversaries, particularly in a 50-year cycle, such as a golden wedding anniversary, golden jubilee, etc.
Great human achievements are frequently rewarded with gold, in the form of medals and decorations. Winners of races and prizes are usually awarded the gold medal (such as the Olympic Games and the Nobel Prize), while many award statues are depicted in gold (such as the Academy Awards, the Golden Globe Awards the Emmy Awards, the Palme d'Or, and the British Academy Film Awards).
Medieval kings were inaugurated under the signs of sacred oil and a golden crown, the latter symbolizing the eternal shining light of heaven and thus a Christian king's divinely inspired authority. Wedding rings are traditionally made of gold; since it is long-lasting and unaffected by the passage of time, it is considered a suitable material for everyday wear as well as a metaphor for the relationship. In Orthodox Christianity, the wedded couple is adorned with a golden crown during the ceremony, an amalgamation of symbolic rites.
The symbolic value of gold varies greatly around the world, even within geographic regions[where?].
[edit] Toxicity

Pure gold is non-toxic and non-irritating when ingested[36] and is sometimes used as a food decoration in the form of gold leaf. It is also a component of the alcoholic drinks Goldschläger, Gold Strike, and Goldwasser. Gold is approved as a food additive in the EU (E175 in the Codex Alimentarius).
Soluble compounds (gold salts) such as potassium gold cyanide, used in gold electroplating, are toxic to the liver and kidneys. There are rare cases of lethal gold poisoning from potassium gold cyanide.[37][38] Gold toxicity can be ameliorated with chelation therapy with an agent such as Dimercaprol.
It was voted Allergen of the Year in 2001 by the American Contact Dermatitis Society.
[edit] See also
[edit] Footnotes
- ^ Relativity in Chemistry
- ^ Schmidbaur, Hubert; Cronje, Stephanie; Djordjevic, Bratislav; Schuster, Oliver (2005). "Understanding gold chemistry through relativity". Chemical Physics 311: 151–161. doi:.
- ^ http://www.utilisegold.com/jewellery_technology/colours/colour_alloys/
- ^ [1]
- ^ BMJ: login required
- ^ Nanoscience and Nanotechnology in Nanomedicine: Hybrid Nanoparticles In Imaging and Therapy of Prostate Cancer - Radiopharmaceutical Sciences Institute, University of Missouri-Columbia
- ^ "The Food Dictionary: Varak". Barron's Educational Services, Inc. 1995. http://www.epicurious.com/cooking/how_to/food_dictionary/entry?id=5061. Retrieved on 2007-05-27.
- ^ Super cars.net. 1994 McLaren F1
- ^ "General Electric Contact Materials". Electrical Contact Catalog (Material Catalog). Tanaka Precious Metals. 2005. http://www.tanaka-precious.com/catalog/material.html. Retrieved on 2007-02-21.
- ^ Nicholas Reeves, Egypt's False Prophet: Akhenaten, Thames & Hudson, p.69
- ^ Mansa Musa - Black History Pages
- ^ "Kingdom of Mali - Primary Source Documents". African studies Center. Boston University. http://www.bu.edu/africa/outreach/materials/handouts/k_o_mali.html. Retrieved on 2008-08-05.
- ^ a b "GOLDSHEET - YEARLY and CUMULATIVE WORLD GOLD PRODUCTION CHARTS". http://www.goldsheetlinks.com/production2.htm. Retrieved on 2006-07-22.
- ^ Moore, Mark A. (2006). "Reed Gold Mine State Historic Site". North Carolina Office of Archives and History. http://www.nchistoricsites.org/Reed/reed.htm. Retrieved on 2008-12-13.
- ^ Garvey, Jane A. (2006). "Road to adventure". Georgia Magazine. http://www.georgiamagazine.org/archives_view.asp?mon=7&yr=2006&ID=1344. Retrieved on 2007-01-23.
- ^ "Environment & Nature News - Bugs grow gold that looks like coral - 28/01/2004". http://www.abc.net.au/science/news/enviro/EnviroRepublish_1032376.htm. Retrieved on 2006-07-22. This is doctoral research undertaken by Frank Reith at the Australian National University, published 2004.
- ^ China now world's largest gold producer; foreign miners at door - MarketWatch
- ^ Beinhoff, Christian ([dead link] – Scholar search). Removal of Barriers to the Abatement of Global Mercury Pollution from Artisanal Gold Mining. http://www.unido.org/file-storage/download/?file_id=10644.
- ^ Dan Plazak, A Hole in the Ground with a Liar at the Top (Salt Lake: Univ. of Utah Press, 2006) (contains a chapter on gold-from seawater swindles)
- ^ "World Gold Council". http://www.invest.gold.org/sites/en/why_gold/demand_and_supply/. Retrieved on 2008-07-04.
- ^ "The Myth of the Gold Supply Deficit". http://www.lewrockwell.com/blumen/blumen14.html. Retrieved on 2009-03-30.
- ^ "World Gold Council > value > research & statistics > statistics > supply and demand statistics". http://www.gold.org/value/stats/statistics/gold_demand/index.html. Retrieved on 2006-07-22.
- ^ "kitco.com: GOLD - London PM Fix 1975 - present (GIF)". http://kitco.com/LFgif/au75-pres.gif. Retrieved on 2006-07-22.
- ^ a b LBMA statistics
- ^ http://kitco.com/LFgif/au3650nyb.gif
- ^ Gold starts 2006 well, but this is not a 25-year high! | Financial Planning
- ^ 2008 London Gold Fixings
- ^ http://upload.wikimedia.org/wikipedia/en/8/84/Longtermdowgoldlogtr1800.png
- ^ Shaw III, C. F. (1999). "Gold-Based Medicinal Agents". Chemical Reviews 99 (9): 2589–2600. doi:.
- ^ Jansen, Martin (2005). "Effects of relativistic motion of electrons on the chemistry of gold and platinum". Solid State Sciences 7 (12): 1464–1474. doi:.
- ^ a b Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Seidel, S.; Seppelt, K. (2000). "Xenon as a Complex Ligand: The Tetra Xenono Gold(II) Cation in AuXe42+(Sb2F11−)2". Science 290 (5489): 117–118. doi:. PMID 11021792.
- ^ Riedel, S.; Kaupp, M. (2006). "Revising the Highest Oxidation States of the 5d Elements: The Case of Iridium(+VII)". Angewandte Chemie International Edition 45 (22): 3708–3711. doi:.
- ^ a b Audi, G. (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A (Atomic Mass Data Center) 729: 3–128. doi:.
- ^ "Symptoms of Gold poisoning - WrongDiagnosis.com". http://www.wrongdiagnosis.com/g/gold_poisoning/symptoms.htm#symptom_list. Retrieved on 2009-03-31.
- ^ S Dierks (May 2005). "Gold MSDS". Electronic Space Products International. http://www.espi-metals.com/msds's/gold.htm.
- ^ I. H. Wright, C. J. Vesey (1986). "Acute poisoning with gold cyanide". Anaesthesia 41 (79): 936–939. doi:.
- ^ Wu, Ming-Ling; Tsai, Wei-Jen; Ger, Jiin; Deng, Jou-Fang; Tsay, Shyh-Haw; Yang, Mo-Hsiung. (2001). "Cholestatic Hepatitis Caused by Acute Gold Potassium Cyanide Poisoning". Clinical toxicology 39 (7): 739–743. doi:.
[edit] Bibliography
- Faulk W, Taylor G (1979) An Immunocolloid Method for the Electron Microscope Immunochemistry 8, 1081–1083.
- Kodak (2006) Toning black-and-white materials. Technical Data/Reference sheet G-23, May 2006.
- Roth J, Bendayan M, Orci L (1980) FITC-Protein A-Gold Complex for Light and Electron Microscopic Immunocytochemistry. Journal of Histochemistry and Cytochemistry 28, 55–57.
- World Gold Council, Jewellery Technology, Jewellery Alloys
- Los Alamos National Laboratory – Gold
[edit] External links
| Wikimedia Commons has media related to: Gold |
| Look up gold in Wiktionary, the free dictionary. |
- Getting Gold 1898 book
- Technical Document on Extraction and Mining of Gold
- Picture in the Element collection from Heinrich Pniok
- WebElements.com — Gold
|
|||||||||||||||||
| Periodic table | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Uub | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||














