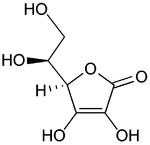
Vitamin C
From Wikipedia, the free encyclopedia
 |
|
 |
|
|
Vitamin C
|
|
| Systematic (IUPAC) name | |
| 2-oxo-L-threo-hexono-1,4- lactone-2,3-enediol or (R)-3,4-dihydroxy-5-((S)- 1,2-dihydroxyethyl)furan-2(5H)-one |
|
| Identifiers | |
| CAS number | |
| ATC code | A |
| PubChem | |
| Chemical data | |
| Formula | C6H8O6 |
| Mol. mass | 176.14 grams per mol |
| Synonyms | L-ascorbate |
| Physical data | |
| Melt. point | 190–192 °C (374–378 °F) decomposes |
| Pharmacokinetic data | |
| Bioavailability | rapid & complete |
| Protein binding | negligible |
| Metabolism | ? |
| Half life | 30 minutes |
| Excretion | renal |
| Therapeutic considerations | |
| Pregnancy cat. |
A |
| Legal status |
general public availability |
| Routes | oral |
Vitamin C or ascorbic acid is an essential nutrient for humans, a large number of higher primate species, a small number of other mammalian species (notably guinea pigs and bats), a few species of birds, and some fish.[1]
Ascorbate (an ion of ascorbic acid) is required for a range of essential metabolic reactions in all animals and plants. It is made internally by almost all organisms, humans being a notable exception. It is widely known that a deficiency in this vitamin causes scurvy in humans.[2][3][4] It is also widely used as a food additive.
The pharmacophore of vitamin C is the ascorbate ion. In living organisms, ascorbate is an anti-oxidant, since it protects the body against oxidative stress,[5] and is a cofactor in several vital enzymatic reactions.[6]
Scurvy has been known since ancient times. People in many parts of the world assumed it was caused by a lack of fresh plant foods. The British Navy adopted the practice of supplementing sailors' diets with lemon juice in 1795[7]. Ascorbic acid was finally isolated by 1933 and synthesized in 1934. The uses and recommended daily intake of vitamin C are matters of on-going debate. A recent meta-analysis of 68 reliable antioxidant supplementation experiments involving a total of 232,606 individuals concluded that consuming additional ascorbate from supplements may not be as beneficial as thought.[8]
[edit] Biological significance
Vitamin C is purely the L-enantiomer of ascorbate; the opposite D-enantiomer has no physiological significance. Both forms are mirror images of the same molecular structure. When L-ascorbate, which is a strong reducing agent, carries out its reducing function, it is converted to its oxidized form, L-dehydroascorbate.[6] L-dehydroascorbate can then be reduced back to the active L-ascorbate form in the body by enzymes and glutathione.[9]. During this process semidehydroascorbic acid radical is formed. Ascorbate free radical reacts poorly with oxygen, and thus, will not create a superoxide. Instead two semidehydroascorbate radicals will react and form one ascorbate and one dehydroascorbate. With the help of glutathione, dehydroxyascorbate is converted back to ascorbate. [10] The presence of Glutathione is crucial since it spares ascorbate and improves antioxidant capacity of blood. [11] Without it dehydroxyascorbate could not convert back to ascorbate.
L-ascorbate is a weak sugar acid structurally related to glucose which naturally occurs either attached to a hydrogen ion, forming ascorbic acid, or to a metal ion, forming a mineral ascorbate.
[edit] Naming
Vitamin C or ascorbic acid is water soluble; also called ‘water soluble C’ [12]. Vitamin, originally called vitamine, means from vital amine. However, not all vitamins contain amine group; hence, the ‘e’ from vitamine was dropped. Previously, there were 2 other vitamin discovered and the third one which follows alphabetical order (ABC) resulted in the name of vitamin C.
[edit] Biosynthesis
The vast majority of animals and plants are able to synthesize their own vitamin C, through a sequence of four enzyme-driven steps, which convert glucose to vitamin C.[6] The glucose needed to produce ascorbate in the liver (in mammals and perching birds) is extracted from glycogen; ascorbate synthesis is a glycogenolysis-dependent process.[13] In reptiles and birds the biosynthesis is carried out in the kidneys.
Among the animals that have lost the ability to synthesise vitamin C are simians (specifically the suborder haplorrhini, which includes humans), guinea pigs, a number of species of passerine birds (but not all of them), and many (perhaps all) major families of bats. These animals all lack the L-gulonolactone oxidase (GULO) enzyme, which is required in the last step of vitamin C synthesis, because they have a defective form of the gene for the enzyme (Pseudogene ΨGULO).[14] Some of these species (including humans) are able to make do with the lower levels available from their diets by recycling oxidised vitamin C.[15]
Most simians consume the vitamin in amounts 10 to 20 times higher than that recommended by governments for humans.[16] This discrepancy constitutes the basis of the controversy on current recommended dietary allowances.
It has been noted that the loss of the ability to synthesize ascorbate strikingly parallels the evolutionary loss of the ability to break down uric acid. Uric acid and ascorbate are both strong reducing agents. This has led to the suggestion that in higher primates, uric acid has taken over some of the functions of ascorbate.[17] Ascorbic acid can be oxidized (broken down) in the human body by the enzyme L-ascorbate oxidase.
An adult goat, a typical example of a vitamin C-producing animal, will manufacture more than 13,000 mg of vitamin C per day in normal health and the biosynthesis will increase "many fold under stress".[18] Trauma or injury has also been demonstrated to use up large quantities of vitamin C in humans.[19] Some microorganisms such as the yeast Saccharomyces cerevisiae have been shown to be able to synthesize vitamin C from simple sugars.[20][21]
[edit] Absorption and Transport
Ascorbic acid is absorbed in the body by both active transport and simple diffusion. Sodium Dependent Active Transport - Sodium-Ascorbate Co-Transporters (SVCTs) and Hexose transporters (GLUTs) are the two transporters required for absorption. SCVT1 and SCVT2 imported the reduced form of ascorbate across plasma membrane [22]. GLUT1, GLUT 3 and GLUT4 are the two glucose transporter and only transfer dehydroxyascorbate form of ascorbate [23]. Thus, dehydroascorbate is absorbed in higher rate than ascorbate.
With at regular intake the absorption rate varies between 70 to 95%. However, the degree of absorption decreases as intake increases. At high intake (12g), human body can absorb ascorbic acid as low as 16%; while, at low intake (<20mg) the absorption rate could reach up to 98% [24].
Biological tissues that accumulate over 100 times the level in blood plasma of vitamin C are the adrenal glands, pituitary, thymus, corpus luteum, and retina.[25] Those with 10 to 50 times the concentration present in blood plasma include the brain, spleen, lung, testicle, lymph nodes, liver, thyroid, small intestinal mucosa, leukocytes, pancreas, kidney and salivary glands.
[edit] Deficiency
Scurvy is an avitaminosis resulting from lack of vitamin C, since without this vitamin, the synthesised collagen is too unstable to perform its function. Scurvy leads to the formation of liver spots on the skin, spongy gums, and bleeding from all mucous membranes. The spots are most abundant on the thighs and legs, and a person with the ailment looks pale, feels depressed, and is partially immobilized. In advanced scurvy there are open, suppurating wounds and loss of teeth and, eventually, death. The human body can store only a certain amount of vitamin C,[26] and so the body soon depletes itself if fresh supplies are not consumed.
It has been shown that smokers who have diets poor in vitamin C are at a higher risk of lung-borne diseases than those smokers who have higher concentrations of vitamin C in the blood. [27]
Nobel prize winner Linus Pauling and Dr. G. C. Willis have asserted that chronic long term low blood levels of vitamin C or Chronic Scurvy is a cause of atherosclerosis.
Western societies generally consume sufficient Vitamin C to prevent scurvy. In 2004 a Canadian Community health survey reported that Canadians of 19 years and above have intakes of vitamin C from food of, 133 mg/d for males and 120 mg/d for females [28] , which is higher than the RDA recommendation.
[edit] History of human understanding

The need to include fresh plant food or raw animal flesh in the diet to prevent disease was known from ancient times. Native peoples living in marginal areas incorporated this into their medicinal lore. For example, spruce needles were used in temperate zones in infusions, or the leaves from species of drought-resistant trees in desert areas. In 1536, the French explorer Jacques Cartier, exploring the St. Lawrence River, used the local natives' knowledge to save his men who were dying of scurvy. He boiled the needles of the arbor vitae tree to make a tea that was later shown to contain 50 mg of vitamin C per 100 grams.[29][30]
Throughout history, the benefit of plant food to survive long sea voyages has been occasionally recommended by authorities. John Woodall, the first appointed surgeon to the British East India Company, recommended the preventive and curative use of lemon juice in his book "The Surgeon's Mate", in 1617. The Dutch writer, Johann Bachstrom, in 1734, gave the firm opinion that "scurvy is solely owing to a total abstinence from fresh vegetable food, and greens; which is alone the primary cause of the disease."
While the earliest documented case of scurvy was described by Hippocrates around the year 400 BC, the first attempt to give scientific basis for the cause of this disease was by a ship's surgeon in the British Royal Navy, James Lind. Scurvy was common among those with poor access to fresh fruit and vegetables, such as remote, isolated sailors and soldiers. While at sea in May 1747, Lind provided some crew members with two oranges and one lemon per day, in addition to normal rations, while others continued on cider, vinegar, sulfuric acid or seawater, along with their normal rations. In the history of science this is considered to be the first occurrence of a controlled experiment comparing results on two populations of a factor applied to one group only with all other factors the same. The results conclusively showed that citrus fruits prevented the disease. Lind published his work in 1753 in his Treatise on the Scurvy[31].

Lind's work was slow to be noticed, partly because he gave conflicting evidence within the book, and partly because the British admiralty saw care for the well-being of crews as a sign of weakness. In addition, fresh fruit was very expensive to keep on board, whereas boiling it down to juice allowed easy storage but destroyed the vitamin (especially if boiled in copper kettles[32]). Ship captains assumed wrongly that Lind's suggestions didn't work because those juices failed to cure scurvy.
It was 1795 before the British navy adopted lemons or lime as standard issue at sea. Limes were more popular as they could be found in British West Indian Colonies, unlike lemons which weren't found in British Dominions, and were therefore more expensive. This practice led to the American use of the nickname "limey" to refer to the British. Captain James Cook had previously demonstrated and proven the principle of the advantages of carrying "Sour krout" on board, by taking his crews to the Hawaiian Islands and beyond without losing any of his men to scurvy[33]. For this otherwise unheard of feat, the British Admiralty awarded him a medal.
The name "antiscorbutic" was used in the eighteenth and nineteenth centuries as general term for those foods known to prevent scurvy, even though there was no understanding of the reason for this. These foods included but were not limited to: lemons, limes, and oranges; sauerkraut, cabbage, malt, and portable soup.
In 1907, Axel Holst and Theodor Frølich, two Norwegian physicians studying beriberi contracted aboard ship's crews in the Norwegian Fishing Fleet, wanted a small test mammal to substitute for the pigeons they used. They fed guinea pigs their test diet, which had earlier produced beriberi in their pigeons, and were surprised when scurvy resulted instead. Until that time scurvy had not been observed in any organism apart from humans, and had been considered an exclusively human disease.
[edit] Discovery of ascorbic acid

In 1912, the Polish-American biochemist Casimir Funk, while researching deficiency diseases, developed the concept of vitamins to refer to the non-mineral micro-nutrients which are essential to health. The name is a portmanteau of "vital", due to the vital role they play biochemically, and "amines" because Funk thought that all these materials were chemical amines. One of the "vitamines" was thought to be the anti-scorbutic factor, long thought to be a component of most fresh plant material.
In 1928 the Arctic anthropologist Vilhjalmur Stefansson attempted to prove his theory of how the Eskimos are able to avoid scurvy with almost no plant food in their diet, despite the disease striking European Arctic explorers living on similar high-meat diets. Stefansson theorised that the natives get their vitamin C from fresh meat that is minimally cooked. Starting in February 1928, for one year he and a colleague lived on an exclusively minimally-cooked meat diet while under medical supervision; they remained healthy. (Later studies done after vitamin C could be quantified in mostly-raw traditional food diets of the Yukon, Inuit, and Métís of the Northern Canada, showed that their daily intake of vitamin C averaged between 52 and 62 mg/day, an amount approximately the dietary reference intake (DRI), even at times of the year when little plant-based food were eaten.)[34]
From 1928 to 1933, the Hungarian research team of Joseph L Svirbely and Albert Szent-Györgyi and, independently, the American Charles Glen King, first isolated the anti-scorbutic factor, calling it "ascorbic acid" for its vitamin activity. Ascorbic acid turned out not to be an amine, nor even to contain any nitrogen. For their accomplishment, Szent-Györgyi was awarded the 1937 Nobel Prize in Medicine "for his discoveries in connection with the biological combustion processes, with special reference to vitamin C and the catalysis of fumaric acid".[35]
Between 1933 and 1934, the British chemists Sir Walter Norman Haworth and Sir Edmund Hirst and, independently, the Polish chemist Tadeus Reichstein, succeeded in synthesizing the vitamin, making it the first to be artificially produced. This made possible the cheap mass-production of what was by then known as vitamin C. Only Haworth was awarded the 1937 Nobel Prize in Chemistry for this work, but the "Reichstein process" retained Reichstein's name.
In 1934 Hoffmann–La Roche became the first pharmaceutical company to mass-produce synthetic vitamin C, under the brand name of Redoxon.
In 1957 the American J.J. Burns showed that the reason some mammals were susceptible to scurvy was the inability of their liver to produce the active enzyme L-gulonolactone oxidase, which is the last of the chain of four enzymes which synthesize vitamin C.[36][37] American biochemist Irwin Stone was the first to exploit vitamin C for its food preservative properties. He later developed the theory that humans possess a mutated form of the L-gulonolactone oxidase coding gene.
In 2008 researchers at the University of Montpellier discovered that in humans and other primates the red blood cells have evolved a mechanism to more efficiently utilize the vitamin C present in the body by recycling oxidized L-dehydroascorbic acid (DHA) back into ascorbic acid which can be reused by the body. The mechanism was not found to be present in mammals that synthesize their own vitamin C.[38]
[edit] Physiological Function
In humans, vitamin C is essential to a healthy diet as well as being a highly effective antioxidant, acting to lessen oxidative stress; a substrate for ascorbate peroxidase[4]; and an enzyme cofactor for the biosynthesis of many important biochemicals. Vitamin C acts as an electron donor for eight different enzymes:[39]
[edit] Collagen, Carnitine, and Tyrosine synthesis, and Microsomal Metabolism
Ascorbic acid bears numerous physiological functions in human body. The functions include synthesis of collagen, carnitine and neurotransmitter, synthesis and catabolism of tyrosine, metabolism of microsome (Gropper, et al., 2005). The main action of ascorbate in most of the synthesis above is functioning as a reducing agent to maintain iron and copper atoms in its reduced state.
- Three participate in collagen hydroxylation.[40][41][42] These reactions add hydroxyl groups to the amino acids proline or lysine in the collagen molecule via prolyl hydroxylase and lysyl hydroxylase, both requiring vitamin C as a cofactor. Hydroxylation allows the collagen molecule to assume its triple helix structure and making vitamin C essential to the development and maintenance of scar tissue, blood vessels, and cartilage.[26]
- 2 are necessary for synthesis of carnitine.[43][44] Carnitine is essential for the transport of fatty acids into mitochondria for ATP generation.
- The remaining three have the following functions in common but do not always do this:
- dopamine beta hydroxylase participates in the biosynthesis of norepinephrine from dopamine.[45][46]
- another enzyme adds amide groups to peptide hormones, greatly increasing their stability.[47][48]
- one modulates tyrosine metabolism.[49][50]
[edit] Antioxidant
Ascorbic acid is well known for its antioxidant activity. Ascorbate acts as reducing agent to reverse oxidation in aqueous solution. When there are more free radicals (Reactive oxygen species) in the body versus antioxidant, human is under the condition called Oxidative stress. [51] Oxidative stress induced diseases encompass cardiovascular diseases, hypertensions, chronic inflammatory diseases and diabetes [52][53] [54] [55] The plasma Ascorbate concentration in oxidative stress patient (less than 45umol/L) measured is lower than healthy individual (61.4-80umol/L) [56]. According to McGregor and Biesalski (2006)[51] increasing plasma ascorbate level may have therapeutic effects in oxidative stress individual. Individual with oxidative stress and healthy individual have different pharmacokinetics of ascorbate.
[edit] Pro-oxidant
Ascorbic acid behaves not only as antioxidant but also as pro-oxidant [51]. Ascorbic acid reduced transition metals, such as Cupric ions (Cu2+) to cuprous (Cu1+) and Ferric ions (Fe3+) to ferrous (Fe2+) during conversion from ascorbate to dehydroxyascorbate In Vitro [57]. This reaction can generate superoxide and other ROS. However, in the body, free transition elements are unlikely to be present while iron and copper is bound to diverse proteins [51]. Recent studies show that intravenous injection of 7.5g of ascorbate daily for 6 days did not increase pro-oxidant markers [58]; thus, ascorbate as a pro-oxidant are unlikely to convert metals to create ROS in vivo.
[edit] Daily requirements
The North American Dietary Reference Intake recommends 90 milligrams per day and no more than 2 grams per day (2000 milligrams per day).[59] Other related species sharing the same inability to produce vitamin C and requiring exogenous vitamin C consume 20 to 80 times this reference intake.[60][61] There is continuing debate within the scientific community over the best dose schedule (the amount and frequency of intake) of vitamin C for maintaining optimal health in humans.[62] It is generally agreed that a balanced diet without supplementation contains enough vitamin C to prevent scurvy in an average healthy adult, while those who are pregnant, smoke tobacco, or are under stress require slightly more.[59]
High doses (thousands of milligrams) may result in diarrhea in healthy adults. Proponents of alternative medicine (specifically orthomolecular medicine)[63] claim the onset of diarrhea to be an indication of where the body’s true vitamin C requirement lies, though this has yet to be clinically verified.
| United States vitamin C recommendations[59] | |
|---|---|
| Recommended Dietary Allowance (adult male) | 90 mg per day |
| Recommended Dietary Allowance (adult female) | 75 mg per day |
| Tolerable Upper Intake Level (adult male) | 2,000 mg per day |
| Tolerable Upper Intake Level (adult female) | 2,000 mg per day |
[edit] Government recommended intakes
Recommendations for vitamin C intake have been set by various national agencies:
- 40 milligrams per day: the United Kingdom's Food Standards Agency[2]
- 45 milligrams per day: the World Health Organization[64]
- 60 mg/day: Health Canada 2007 [3]
- 60–95 milligrams per day: United States' National Academy of Sciences.[59]
The United States defined Tolerable Upper Intake Level for a 25-year-old male is 2,000 milligrams per day.
[edit] Alternative recommendations on intakes
Some independent researchers have calculated the amount needed for an adult human to achieve similar blood serum levels as vitamin C synthesising mammals as follows:
- 400 milligrams per day: the Linus Pauling Institute.[65]
- 500 milligrams per 12 hours: Professor Roc Ordman, from research into biological free radicals.[66]
- 3,000 milligrams per day (or up to 30,000 mg during illness): the Vitamin C Foundation.[67]
- 6,000–12,000 milligrams per day: Thomas E. Levy, Colorado Integrative Medical Centre.[68]
- 6,000–18,000 milligrams per day: Linus Pauling's personal use.[69]
[edit] Vitamin C megadosage
Several individuals and organizations advocate large doses of vitamin C based on in vitro and retrospective studies,[70] although large, randomized clinical trials on the effects of high doses on the general population have never taken place. Individuals who have recommended intake well in excess of the current Dietary Reference Intake (DRI) include Robert Cathcart, Ewan Cameron, Steve Hickey, Irwin Stone, Matthias Rath and Linus Pauling. Arguments for megadosage are based on the diets of closely related apes and the likely diet of pre-historical humans, and that most mammals synthesize vitamin C rather than relying on dietary intake.
Stone[71] and Pauling[61] calculated, based on the diet of our primate cousins[60] (similar to what our common ancestors are likely to have consumed when the gene mutated), that the optimum daily requirement of vitamin C is around 2,300 milligrams for a human requiring 2,500 kcal a day. Pauling also criticized the established RDA as sufficient to prevent scurvy, but not necessarily the dosage for optimal health.[69]
[edit] Therapeutic uses
Vitamin C is necessary for the treatment and prevention of scurvy. Scurvy is commonly comorbid with other diseases of malnutrition; sufficient vitamin C to prevent scurvy occurs in most diets in industrialized nations.[72][73][74]
Vitamin C functions as an antioxidant. Adequate intake is necessary for health, but supplementation is probably not necessary in most cases.[75][76][77][78]
Based on animal and epidemiological models, high doses of vitamin C may have "protective effects" on lead-induced nerve and muscle abnormalities,[79] especially in smokers.[80][81]
Dehydroascorbic acid, the main form of oxidized vitamin C in the body, may reduce neurological deficits and mortality following stroke due to its ability to cross the blood-brain barrier, while "the antioxidant ascorbic acid (AA) or vitamin C does not penetrate the blood-brain barrier".[82]
Higher vitamin C intake reduces serum uric acid levels, and is associated with lower incidence of gout.[83]
Vitamin C has also been promoted as efficacious against a vast array of diseases and syndromes. Variously, adequate dietary intake, oral megadose, or intravenous injection may be required for the purported benefits. These disorders include: the common cold,[84][85] pneumonia,[86] bird flu[87], SARS,[87] heart disease,[88][85] AIDS,[89][90] autism,[91] low sperm count,[92] age-related macular degeneration,[93][94] altitude sickness,[95] pre-eclampsia,[96] amyotrophic lateral sclerosis,[97] asthma,[98] tetanus,[99] and cancer.[100][101][102][103] These uses are poorly supported by the evidence, and sometimes contraindicated.[104][105][106][107][108][109]
[edit] Testing for ascorbate levels in the body
Simple tests use DCPIP to measure the levels of vitamin C in the urine and in serum or blood plasma. However these reflect recent dietary intake rather than the level of vitamin C in body stores.[6] Reverse phase high performance liquid chromatography is used for determining the storage levels of vitamin C within lymphocytes and tissue.
It has been observed that while serum or blood plasma levels follow the circadian rhythm or short term dietary changes, those within tissues themselves are more stable and give a better view of the availability of ascorbate within the organism. However, very few hospital laboratories are adequately equipped and trained to carry out such detailed analyses, and require samples to be analyzed in specialized laboratories.[110][111]
[edit] Adverse effects
[edit] Common side-effects
Relatively large doses of vitamin C may cause indigestion, particularly when taken on an empty stomach.
When taken in large doses, vitamin C causes diarrhea in healthy subjects. In one trial, doses up to 6 grams of ascorbic acid were given to 29 infants, 93 children of preschool and school age, and 20 adults for more than 1400 days. With the higher doses, toxic manifestations were observed in five adults and four infants. The signs and symptoms in adults were nausea, vomiting, diarrhea, flushing of the face, headache, fatigue and disturbed sleep. The main toxic reactions in the infants were skin rashes.[112]
[edit] Possible side-effects
As vitamin C enhances iron absorption[113], iron poisoning can become an issue to people with rare iron overload disorders, such as haemochromatosis. A genetic condition that results in inadequate levels of the enzyme glucose-6-phosphate dehydrogenase (G6PD) can cause sufferers to develop hemolytic anemia after ingesting specific oxidizing substances, such as very large dosages of vitamin C.[114]
There is a longstanding belief among the mainstream medical community that vitamin C causes kidney stones, which is based on little science.[115] Although recent studies have found a relationship[116] a clear relationship between excess ascorbic acid intake and kidney stone formation has not been generally established. [117]
In a study conducted on rats, during the first month of pregnancy, high doses of vitamin C may suppress the production of progesterone from the corpus luteum.[118] Progesterone, necessary for the maintenance of a pregnancy, is produced by the corpus luteum for the first few weeks, until the placenta is developed enough to produce its own source. By blocking this function of the corpus luteum, high doses of vitamin C (1000+ mg) are theorized to induce an early miscarriage. In a group of spontaneously aborting women at the end of the first trimester, the mean values of vitamin C were significantly higher in the aborting group. However, the authors do state: 'This could not be interpreted as an evidence of causal association.'[119] However, in a previous study of 79 women with threatened, previous spontaneous, or habitual abortion, Javert and Stander (1943) had 91% success with 33 patients who received vitamin C together with bioflavonoids and vitamin K (only three abortions), whereas all of the 46 patients who did not receive the vitamins aborted. [120]
Recent rat and human studies suggest that adding Vitamin C supplements to an exercise training program can cause a decrease in mitochondria production, hampering endurance capacity.[121]
[edit] Chance of overdose
As discussed previously, vitamin C exhibits remarkably low toxicity. The LD50 (the dose that will kill 50% of a population) in rats is generally accepted to be 11.9 grams per kilogram of body weight when taken orally.[32] The LD50 in humans remains unknown, owing to medical ethics that preclude experiments which would put patients at risk of harm. However, as with all substances tested in this way, the LD50 is taken as a guide to its toxicity in humans and no data to contradict this has been found.
[edit] Natural and artificial dietary sources

The richest natural sources are fruits and vegetables, and of those, the Kakadu plum and the camu camu fruit contain the highest concentration of the vitamin. It is also present in some cuts of meat, especially liver. Vitamin C is the most widely taken nutritional supplement and is available in a variety of forms, including tablets, drink mixes, crystals in capsules or naked crystals.
Vitamin C is absorbed by the intestines using a sodium-ion dependent channel. It is transported through the intestine via both glucose-sensitive and glucose-insensitive mechanisms. The presence of large quantities of sugar either in the intestines or in the blood can slow absorption.[122]
[edit] Plant sources
While plants are generally a good source of vitamin C, the amount in foods of plant origin depends on: the precise variety of the plant, the soil condition, the climate in which it grew, the length of time since it was picked, the storage conditions, and the method of preparation.[123]
The following table is approximate and shows the relative abundance in different raw plant sources.[124][125][126] As some plants were analyzed fresh while others were dried (thus, artifactually increasing concentration of individual constituents like vitamin C), the data are subject to potential variation and difficulties for comparison. The amount is given in milligrams per 100 grams of fruit or vegetable and is a rounded average from multiple authoritative sources:
| Plant source | Amount (mg / 100g) |
|---|---|
| Kakadu plum | 3100 |
| Camu Camu | 2800 |
| Rose hip | 2000 |
| Acerola | 1600 |
| Seabuckthorn | 695 |
| Jujube | 500 |
| Indian gooseberry | 445 |
| Baobab | 400 |
| Blackcurrant | 200 |
| Red pepper | 190 |
| Parsley | 130 |
| Guava | 100 |
| Kiwifruit | 90 |
| Broccoli | 90 |
| Loganberry | 80 |
| Redcurrant | 80 |
| Brussels sprouts | 80 |
| Wolfberry (Goji) | 73 † |
| Lychee | 70 |
| Cloudberry | 60 |
| Elderberry | 60 |
| Persimmon | 60 |
† average of 3 sources; dried
| Plant source | Amount (mg / 100g) |
|---|---|
| Papaya | 60 |
| Strawberry | 60 |
| Orange | 50 |
| Lemon | 40 |
| Melon, cantaloupe | 40 |
| Cauliflower | 40 |
| Garlic | 31 |
| Grapefruit | 30 |
| Raspberry | 30 |
| Tangerine | 30 |
| Mandarin orange | 30 |
| Passion fruit | 30 |
| Spinach | 30 |
| Cabbage raw green | 30 |
| Lime | 30 |
| Mango | 28 |
| Blackberry | 21 |
| Potato | 20 |
| Melon, honeydew | 20 |
| Cranberry | 13 |
| Tomato | 10 |
| Blueberry | 10 |
| Pineapple | 10 |
| Pawpaw | 10 |
| Plant source | Amount (mg / 100g) |
|---|---|
| Grape | 10 |
| Apricot | 10 |
| Plum | 10 |
| Watermelon | 10 |
| Banana | 9 |
| Carrot | 9 |
| Avocado | 8 |
| Crabapple | 8 |
| Persimmon - fresh | 7 |
| Cherry | 7 |
| Peach | 7 |
| Apple | 6 |
| Asparagus | 6 |
| Beetroot | 5 |
| Chokecherry | 5 |
| Pear | 4 |
| Lettuce | 4 |
| Cucumber | 3 |
| Eggplant | 2 |
| Raisin | 2 |
| Fig | 2 |
| Bilberry | 1 |
| Horned melon | 0.5 |
| Medlar | 0.3 |
[edit] Animal sources
The overwhelming majority of species of animals and plants synthesise their own vitamin C, making some, but not all, animal products, sources of dietary vitamin C.
Vitamin C is most present in the liver and least present in the muscle. Since muscle provides the majority of meat consumed in the western human diet, animal products are not a reliable source of the vitamin. Vitamin C is present in mother's milk and, in lower amounts, in raw cow's milk, with pasteurized milk containing only trace amounts.[127] All excess vitamin C is disposed of through the urinary system.
The following table shows the relative abundance of vitamin C in various foods of animal origin, given in milligram of vitamin C per 100 grams of food:
| Animal Source | Amount (mg / 100g) |
|---|---|
| Calf liver (raw) | 36 |
| Beef liver (raw) | 31 |
| Oysters (raw) | 30 |
| Cod roe (fried) | 26 |
| Pork liver (raw) | 23 |
| Lamb brain (boiled) | 17 |
| Chicken liver (fried) | 13 |
| Animal Source | Amount (mg / 100g) |
|---|---|
| Lamb liver (fried) | 12 |
| Calf adrenals (raw) | 11 [128] |
| Lamb heart (roast) | 11 |
| Lamb tongue (stewed) | 6 |
| Human milk (fresh) | 4 |
| Goat milk (fresh) | 2 |
| Cow milk (fresh) | 2 |
[edit] Food preparation
Vitamin C chemically decomposes under certain conditions, many of which may occur during the cooking of food. Normally, boiling water at 100°C is not hot enough to cause any significant destruction of the nutrient, which only decomposes at 190°C,[32] despite popular opinion. However, pressure cooking, roasting, frying and grilling food is more likely to reach the decomposition temperature of vitamin C. Longer cooking times also add to this effect, as will copper food vessels, which catalyse the decomposition.[32]
Another cause of vitamin C being lost from food is leaching, where the water-soluble vitamin dissolves into the cooking water, which is later poured away and not consumed. However, vitamin C doesn't leach in all vegetables at the same rate; research shows broccoli seems to retain more than any other.[129] Research has also shown that fresh-cut fruits don't lose significant nutrients when stored in the refrigerator for a few days.[130]
[edit] Vitamin C supplements

Vitamin C is the most widely taken dietary supplement.[131] It is available in many forms including caplets, tablets, capsules, drink mix packets, in multi-vitamin formulations, in multiple antioxidant formulations, and crystalline powder. Timed release versions are available, as are formulations containing bioflavonoids such as quercetin, hesperidin and rutin. Tablet and capsule sizes range from 25 mg to 1500 mg. Vitamin C (as ascorbic acid) crystals are typically available in bottles containing 300 g to 1 kg of powder (a teaspoon of vitamin C crystals equals 5,000 mg).
[edit] Artificial modes of synthesis
Vitamin C is produced from glucose by two main routes. The Reichstein process, developed in the 1930s, uses a single pre-fermentation followed by a purely chemical route. The modern two-step fermentation process, originally developed in China in the 1960s, uses additional fermentation to replace part of the later chemical stages. Both processes yield approximately 60% vitamin C from the glucose feed.[132]
Research is underway at the Scottish Crop Research Institute in the interest of creating a strain of yeast that can synthesise vitamin C in a single fermentation step from galactose, a technology expected to reduce manufacturing costs considerably.[20]
World production of synthesised vitamin C is currently estimated at approximately 110,000 tonnes annually. Main producers have been BASF/Takeda, DSM, Merck and the China Pharmaceutical Group Ltd. of the People's Republic of China. China is slowly becoming the major world supplier as its prices undercut those of the US and European manufacturers.[133] By 2008 only the DSM plant in Scotland remained operational outside the strong price competition from China. [134] The world price of vitamin C rose sharply in 2008 partly as a result of rises in basic food prices but also in anticipation of a stoppage of the two Chinese plants, situated at Shijiazhuang near Beijing, as part of a general shutdown of polluting industry in China over the period of the Olympic games.[135]
[edit] Fortification
In Addition of Vitamins and Minerals to Food, 2005: Health Canada's Proposed Policy and Implementation Plans [136]. Ascorbate is categorized in ‘Risk Category A nutrients’ which for ‘‘those nutrients for which a UL was set but with a wide margin of safe intake; and those nutrients with a narrow margin of safety, but non-serious critical adverse effects’’. The level of additions set by health canada are Minimum of 3 mg or 5 % of RDI to be able to claim as "Source" and maximum fortification of 12 mg (20 % of RDI) to be claimed "Excellent Source".
[edit] See also
[edit] References
- ^ McCluskey, Elwood S. (1985). "Which Vertebrates Make Vitamin C?" (PDF). Origins 12 (2): 96–100. http://www.grisda.org/origins/12096.pdf.
- ^ a b "Vitamin C". Food Standards Agency (UK). http://www.eatwell.gov.uk/healthydiet/nutritionessentials/vitaminsandminerals/vitaminc/. Retrieved on 2007-02-19.
- ^ "Vitamin C". University of Maryland Medical Center. January 2007. http://www.umm.edu/ency/article//002404.htm. Retrieved on 2008-03-31.
- ^ a b Higdon, Jane, Ph.D. (2006-01-31). "Vitamin C". Oregon State University, Micronutrient Information Center. http://lpi.oregonstate.edu/infocenter/vitamins/vitaminC/. Retrieved on 2007-03-07.
- ^ Padayatty S, Katz A, Wang Y, Eck P, Kwon O, Lee J, Chen S, Corpe C, Dutta A, Dutta S, Levine M (2003). "Vitamin C as an Antioxidant: evaluation of its role in disease prevention" (PDF). J Am Coll Nutr 22 (1): 18–35. PMID 12569111. http://www.jacn.org/cgi/reprint/22/1/18.pdf.
- ^ a b c d "Vitamin C – Risk Assessment" (PDF). UK Food Standards Agency. http://www.food.gov.uk/multimedia/pdfs/evm_c.pdf. Retrieved on 2007-02-19.
- ^ , Wilson LG. The Clinical Definition of Scurvy and the Discovery of Vitamin C J Hist of Med1975;40-60.
- ^ Bjelakovic G, et al (2007). "Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis". JAMA 297 (8): 842–57. doi:. PMID 17327526.
- ^ Meister A (1994). "Glutathione-ascorbic acid antioxidant system in animals" (PDF). J Biol Chem 269 (13): 9397–400. PMID 8144521. http://www.jbc.org/cgi/reprint/269/13/9397.pdf.
- ^ , Nualart FJ, Rivas CI, Montecinos VP, et al. Recycling of vitamin C by a bystander effect. J Biol Chem 2003; 278:10128–10133.
- ^ Gropper SS, Smith JL, Grodd JL. 2004. Advanced Nutrition and Human Metabolism. Fourth Edition. Thomson Wadsworth, Belmont, CA. USA. pp. 260-275.
- ^ Drummond JC, Note on the role of the anti-scorbutic factor in nutrition. Biochem.J.,1919; 77-80.
- ^ Bánhegyi G, Mándl J (2001). "The hepatic glycogenoreticular system". Pathol Oncol Res 7 (2): 107–10. PMID 11458272.
- ^ Harris, J. Robin (1996). Ascorbic Acid: Subcellular Biochemistry. Springer. pp. 35. ISBN 0306451484. OCLC 34307319 46753025.
- ^ How Humans Make Up For An 'Inborn' Vitamin C Deficiency. http://www.sciencedaily.com/releases/2008/03/080320120726.htm.
- ^ Milton K (June 1999). "Nutritional characteristics of wild primate foods: do the diets of our closest living relatives have lessons for us?". Nutrition 15 (6): 488–98. doi:. PMID 10378206. http://linkinghub.elsevier.com/retrieve/pii/S0899-9007(99)00078-7.
- ^ Proctor P (1970). "Similar functions of uric acid and ascorbate in man?". Nature 228 (5274): 868. doi:. PMID 5477017.
- ^ Stone, Irwin (July 16, 1978). "Eight Decades of Scurvy. The Case History of a Misleading Dietary Hypothesis". http://www.seanet.com/~alexs/ascorbate/197x/stone-i-orthomol_psych-1979-v8-n2-p58.htm. Retrieved on 2007-04-06. "Biochemical research in the 1950’s showed that the lesion in scurvy is the absence of the enzyme, L-Gulonolactone oxidase (GLO) in the human liver (Burns, 1959). This enzyme is the last enzyme in a series of four which converts blood sugar, glucose, into ascorbate in the mammalian liver. This liver metabolite, ascorbate, is produced in an unstressed goat for instance, at the rate of about 13,000 mg per day per 150 pounds body weight (Chatterjee, 1973). A mammalian feedback mechanism increases this daily ascorbate production many fold under stress (Subramanian et al., 1973)"
- ^ C. Long, et al. (2003). "Ascorbic acid dynamics in the seriously ill and injured". Journal of Surgical Research 109 (2): 144–148. doi:.
- ^ a b R.D. Hancock & R. Viola. "Ascorbic acid biosynthesis in higher plants and micro-organisms" (PDF). Scottish Crop Research Institute. http://www.scri.ac.uk/scri/file/individualreports/2002/20Ascorb.pdf. Retrieved on 2007-02-20.
- ^ Hancock RD, Galpin JR, Viola R.. "Biosynthesis of L-ascorbic acid (vitamin C) by Saccharomyces cerevisiae" (PDF). FEMS Microbiol Lett. 186 (2): 245–50. PMID 10802179. http://www.sciencedirect.com/science?_ob=MImg&_imagekey=B6T2W-405SX5F-M-3&_cdi=4929&_user=308069&_orig=search&_coverDate=05%2F05%2F2000&_sk=998139997&view=c&wchp=dGLbVzb-zSkzS&md5=d38569a901eecfe57b39eafa00a1d738&ie=/sdarticle.pdf. Retrieved on 2007-02-19.
- ^ Savini, I., Rossi, A., Pierro, C., et al. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids 2008; 34: 347–355
- ^ Rumsey SC, Kwon O, Xu GW, et al. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem 1997; 272:18982–18989.
- ^ Levine M, et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 1996; 93:3704–3709.
- ^ Hediger MA (May 2002). "New view at C". Nat. Med. 8 (5): 445–6. doi:. PMID 11984580. http://www.nature.com/nm/journal/v8/n5/full/nm0502-445.html.
- ^ a b MedlinePlus Encyclopedia Ascorbic acid
- ^ "The influence of smoking on vitamin C status in adults". BBC news and Cambridge University. 2000-09-31. http://news.bbc.co.uk/2/hi/health/901196.stm. Retrieved on 2007-12-12.
- ^ [1]Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004)
- ^ "Jacques Cartier's Second Voyage - 1535 - Winter & Scurvy". http://www3.sympatico.ca/goweezer/canada/z00cartier3.htm. Retrieved on 2007-02-25.
- ^ Martini E. (June 2002). "Jacques Cartier witnesses a treatment for scurvy". Vesalius. PMID 12422875.
- ^ Lind, James (1753). A Treatise of the Scurvy. London: A. Millar.
- ^ a b c d "Safety (MSDS) data for ascorbic acid". Oxford University. 2005-10-09. http://physchem.ox.ac.uk/MSDS/AS/ascorbic_acid.html. Retrieved on 2007-02-21.
- ^ Cook, James; Philip Edwards (1999). The Journals of Captain Cook. Penguin Books. pp. 38. ISBN 0140436472. OCLC 42445907.
- ^ Kuhnlein HV, Receveur O, Soueida R, Egeland GM (01 June 2004). "Arctic indigenous peoples experience the nutrition transition with changing dietary patterns and obesity". J Nutr. 134 (6): 1447–53. PMID 15173410. http://jn.nutrition.org/cgi/content/full/134/6/1447.
- ^ "Pitt History - 1932: Charles Glen King". University of Pittsburgh. http://www.pitt.edu/history/1932.html. Retrieved on 2007-02-21. "In recognition of this medical breakthrough, some scientists believe that King deserved a Nobel Prize."
- ^ BURNS JJ, EVANS C (01 December 1956). "The synthesis of L-ascorbic acid in the rat from D-glucuronolactone and L-gulonolactone". J Biol Chem. 223 (2): 897–905. PMID 13385237. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=13385237.
- ^ Burns JJ, Moltz A, Peyser P (December 1956). "Missing step in guinea pigs required for the biosynthesis of L-ascorbic acid". Science 124 (3232): 1148–9. doi:. PMID 13380431. http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=13380431.
- ^ "How Humans Make Up For An 'Inborn' Vitamin C Deficiency". ScienceDaily. Cell Press. March 21, 2008. http://www.sciencedaily.com/releases/2008/03/080320120726.htm. Retrieved on 2009-02-24.
- ^ Levine M, Rumsey SC, Wang Y, Park JB, Daruwala R (2000). "Vitamin C". in Stipanuk MH. Biochemical and physiological aspects of human nutrition. Philadelphia: W.B. Saunders. pp. 541–67. ISBN 0-7216-4452-X.
- ^ Prockop DJ, Kivirikko KI (1995). "Collagens: molecular biology, diseases, and potentials for therapy". Annu Rev Biochem. 64: 403–34. doi:. PMID 7574488.
- ^ Peterkofsky B (01 December 1991). "Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy". Am J Clin Nutr. 54 (6 Suppl): 1135S–1140S. PMID 1720597. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=1720597.
- ^ Kivirikko KI, Myllylä R (1985). "Post-translational processing of procollagens". Ann. N. Y. Acad. Sci. 460: 187–201. doi:. PMID 3008623. http://www3.interscience.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=0077-8923&date=1985&volume=460&spage=187.
- ^ Rebouche CJ (1991). "Ascorbic acid and carnitine biosynthesis" (PDF). Am J Clin Nutr 54 (6 Suppl): 1147S–1152S. PMID 1962562. http://www.ajcn.org/cgi/reprint/54/6/1147S.pdf.
- ^ Dunn WA, Rettura G, Seifter E, Englard S (1984). "Carnitine biosynthesis from gamma-butyrobetaine and from exogenous protein-bound 6-N-trimethyl-L-lysine by the perfused guinea pig liver. Effect of ascorbate deficiency on the in situ activity of gamma-butyrobetaine hydroxylase" (PDF). J Biol Chem 259 (17): 10764–70. PMID 6432788. http://www.jbc.org/cgi/reprint/259/17/10764.pdf.
- ^ Levine M, Dhariwal KR, Washko P, et al (1992). "Ascorbic acid and reaction kinetics in situ: a new approach to vitamin requirements". J Nutr Sci Vitaminol. Spec No: 169–72. PMID 1297733.
- ^ Kaufman S (1974). "Dopamine-beta-hydroxylase". J Psychiatr Res 11: 303–16. doi:. PMID 4461800. http://linkinghub.elsevier.com/retrieve/pii/0022-3956(74)90112-5.
- ^ Eipper BA, Milgram SL, Husten EJ, Yun HY, Mains RE (01 April 1993). "Peptidylglycine alpha-amidating monooxygenase: a multifunctional protein with catalytic, processing, and routing domains". Protein Sci. 2 (4): 489–97. PMID 8518727. PMC: 2142366. http://www.proteinscience.org/cgi/pmidlookup?view=long&pmid=8518727.
- ^ Eipper BA, Stoffers DA, Mains RE (1992). "The biosynthesis of neuropeptides: peptide alpha-amidation". Annu Rev Neurosci. 15: 57–85. doi:. PMID 1575450.
- ^ Englard S, Seifter S (1986). "The biochemical functions of ascorbic acid". Annu. Rev. Nutr. 6: 365–406. doi:. PMID 3015170.
- ^ Lindblad B, Lindstedt G, Lindstedt S (December 1970). "The mechanism of enzymic formation of homogentisate from p-hydroxyphenylpyruvate". J Am Chem Soc. 92 (25): 7446–9. doi:. PMID 5487549.
- ^ a b c d McGregor GP, Biesalski HK. Rationale and impact of vitamin C in clinical nutrition. Curr Opin Clin Nutr Metab Care 2006; 9:697–703
- ^ Kelly FJ. Use of antioxidants in the prevention and treatment of disease. J Int Fed Clin Chem 1998; 10:21–23
- ^ Mayne ST. Antioxidant nutrients and chronic disease: use of biomarkers of exposure and oxidative stress status in epidemiologic research. J Nutr 2003; 133 (Suppl 3):933S–940S
- ^ Tak PP, Zvaifler NJ, Green DR, Firestein GS. Rheumatoid arthritis and p53: how oxidative stress might alter the course of inflammatory diseases. Immunol Today 2000; 21:78–82.
- ^ Goodyear-Bruch C, Pierce JD. Oxidative stress in critically ill patients. Am J Crit Care 2002; 11:543–551; quiz 552–543.
- ^ Schorah CJ, Downing C, Piripitsi A, et al. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am J Clin Nutr 1996; 63:760–765.
- ^ Satoh K, Sakagami H. Effect of metal ions on radical intensity and cytotoxic activity of ascorbate. Anticancer Res 1997; 17:1125–1129.
- ^ Muhlhofer A, Mrosek S, Schlegel B, et al. High-dose intravenous vitamin C is not associated with an increase of pro-oxidative biomarkers. Eur J Clin Nutr 2004; 58:1151–1158.
- ^ a b c d "US Recommended Dietary Allowance (RDA)" (PDF). http://www.iom.edu/Object.File/Master/7/296/webtablevitamins.pdf. Retrieved on 2007-02-19.
- ^ a b Milton K (2003). "Micronutrient intakes of wild primates: are humans different?" (PDF). Comp Biochem Physiol a Mol Integr Physiol 136 (1): 47–59. doi:. PMID 14527629. http://nature.berkeley.edu/miltonlab/pdfs/kmilton_micronutrient.pdf.
- ^ a b Pauling, Linus (1970). "Evolution and the need for ascorbic acid". Proc Natl Acad Sci U S a 67 (4): 1643–8. doi:. PMID 5275366.
- ^ "Linus Pauling Vindicated; Researchers Claim RDA For Vitamin C is Flawed". PR Newswire. 6 July 2004. http://www.prnewswire.com/cgi-bin/stories.pl?ACCT=109&STORY=/www/story/07-06-2004/0002204911. Retrieved on 2007-02-20.
- ^ Cathcart, Robert (1994). "Vitamin C, Titrating To Bowel Tolerance, Anascorbemia, and Acute Induced Scurvy". Orthomed. http://www.orthomed.com/titrate.htm. Retrieved on 2007-02-22.
- ^ "Vitamin and mineral requirements in human nutrition, 2nd edition" (PDF). World Health Organization. 2004. http://whqlibdoc.who.int/publications/2004/9241546123_chap7.pdf. Retrieved on 2007-02-20.
- ^ Higdon, Jane. "Linus Pauling Institute Recommendations". Oregon State University. http://lpi.oregonstate.edu/infocenter/vitamins/vitaminC/index.html#lpi_recommend. Retrieved on 2007-04-11.
- ^ Roc Ordman. "The Scientific Basis Of The Vitamin C Dosage Of Nutrition Investigator". Beloit College. http://www.beloit.edu/~nutritio/vitCdose.htm. Retrieved on 2007-02-22.
- ^ "Vitamin C Foundation's RDA". http://www.vitamincfoundation.org/vitcrda.htm. Retrieved on 2007-02-12.
- ^ Levy, Thomas E. (2002). Vitamin C Infectious Diseases, & Toxins. Xlibris. ISBN 1401069630. OCLC 123353969. Chapter 5 - Vitamin C optidosing.
- ^ a b Pauling, Linus (1986). How to Live Longer and Feel Better. W. H. Freeman and Company. ISBN 0-380-70289-4. OCLC 154663991 15690499.
- ^ Douglas RM, Hemilä H (2005). "Vitamin C for Preventing and Treating the Common Cold". PLoS Medicine 2 (6): e168. doi:.
- ^ Stone, Irwin (1972). The Healing Factor: Vitamin C Against Disease. Grosset and Dunlap. ISBN 0-448-11693-6. OCLC 3967737. http://www.vitamincfoundation.org/stone/.
- ^ WHO (June 4, 2001) (PDF). Area of work: nutrition. Progress report 2000. http://www.who.int//mipfiles/2299/MIP_01_APR_SDE_3.en.pdf.
- ^ Olmedo JM, Yiannias JA, Windgassen EB, Gornet MK (August 2006). "Scurvy: a disease almost forgotten". Int. J. Dermatol. 45 (8): 909–13. doi:. PMID 16911372.
- ^ Velandia B, Centor RM, McConnell V, Shah M (August 2008). "Scurvy is still present in developed countries". J Gen Intern Med 23 (8): 1281–4. doi:. PMID 18459013.
- ^ Shenkin A (2006). "The key role of micronutrients". Clin Nutr 25 (1): 1–13. doi:. PMID 16376462.
- ^ Woodside J, McCall D, McGartland C, Young I (2005). "Micronutrients: dietary intake v. supplement use". Proc Nutr Soc 64 (4): 543–53. doi:. PMID 16313697.
- ^ Stanner SA, Hughes J, Kelly CN, Buttriss J (2004). "A review of the epidemiological evidence for the 'antioxidant hypothesis'". Public Health Nutr 7 (3): 407–22. doi:. PMID 15153272.
- ^ Rivers, Jerry M (1987), "Safety of High-level Vitamin C Ingestion", Annals of the New York Academy of Sciences 498: 445, doi:
- ^ Hasan MY, Alshuaib WB, Singh S, Fahim MA (2003). "Effects of ascorbic acid on lead induced alterations of synaptic transmission and contractile features in murine dorsiflexor muscle". Life Sci. 73 (8): 1017–25. doi:. PMID 12818354.
- ^ Dawson E, Evans D, Harris W, Teter M, McGanity W (1999). "The effect of ascorbic acid supplementation on the blood lead levels of smokers". J Am Coll Nutr 18 (2): 166–70. PMID 10204833.
- ^ Simon JA, Hudes ES (1999). "Relationship of ascorbic acid to blood lead levels". JAMA 281 (24): 2289–93. doi:. PMID 10386552.
- ^ Huang J, Agus DB, Winfree CJ, Kiss S, Mack WJ, McTaggart RA, Choudhri TF, Kim LJ, Mocco J, Pinsky DJ, Fox WD, Israel RJ, Boyd TA, Golde DW, Connolly ES Jr. (2001). "Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke". Proceedings of the National Academy of Sciences 98 (20): 11720–4. doi:. PMID 11573006.
- ^ Choi, MD, DrPH, Hyon K.; Xiang Gao, MD, PhD; Gary Curhan, MD, ScD (March 9, 2009). "Vitamin C Intake and the Risk of Gout in Men". Archives of Internal Medicine. Vol. 169 (No. 5): 502-507. http://archinte.ama-assn.org/cgi/content/abstract/169/5/502.
- ^ Douglas RM, Hemilä H, Chalker E, Treacy B (2007). "Vitamin C for preventing and treating the common cold". Cochrane Database Syst Rev (3): CD000980. doi:. PMID 17636648.
- ^ a b Rath MW, Pauling LC. U.S. Patent 5,278,189 Prevention and treatment of occlusive cardiovascular disease with ascorbate and substances that inhibit the binding of lipoprotein(a). USPTO. 11 Jan 1994.
- ^ Hemilä H, Louhiala P (2007). "Vitamin C for preventing and treating pneumonia". Cochrane Database Syst Rev (1): CD005532. doi:. PMID 17253561.
- ^ a b Hemilä H (2003). "Vitamin C and SARS coronavirus". J Antimicrob Chemother 52 (6): 1049–50. doi:. PMID 14613951.
- ^ Vitamin and Mineral Supplements from the American Heart Association
- ^ "Nigeria: Vitamin C Can Suppress HIV/Aids Virus". allAfrica.com. 2006-05-22. http://allafrica.com/stories/200605220885.html. Retrieved on 2006-06-16.
- ^ Boseley, Sarah (2005-05-14). "Discredited doctor's 'cure' for Aids ignites life-and-death struggle in South Africa". The Guardian. http://www.guardian.co.uk/aids/story/0,7369,1483821,00.html. Retrieved on 2007-02-21.
- ^ Levy SE, Hyman SL (2005). "Novel treatments for autistic spectrum disorders". Ment Retard Dev Disabil Res Rev 11 (2): 131–42. doi:. PMID 15977319.
- ^ Akmal M, Qadri J, Al-Waili N, Thangal S, Haq A, Saloom K (2006). "Improvement in human semen quality after oral supplementation of vitamin C". J Med Food 9 (3): 440–2. doi:. PMID 17004914.
- ^ Evans JR (2006). "Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration". Cochrane Database Syst Rev (2): CD000254. doi:. PMID 16625532.
- ^ Evans J (June 2008). "Antioxidant supplements to prevent or slow down the progression of AMD: a systematic review and meta-analysis". Eye 22 (6): 751–60. doi:. PMID 18425071.
- ^ Baillie JK, Thompson AA, Irving JB, et al (March 2009). "Oral antioxidant supplementation does not prevent acute mountain sickness: double blind, randomized placebo-controlled trial". QJM. doi:. PMID 19273551.
- ^ Rumbold A, Duley L, Crowther CA, Haslam RR (2008). "Antioxidants for preventing pre-eclampsia". Cochrane Database Syst Rev (1): CD004227. doi:. PMID 18254042.
- ^ Orrell RW, Lane RJ, Ross M (2007). "Antioxidant treatment for amyotrophic lateral sclerosis / motor neuron disease". Cochrane Database Syst Rev (1): CD002829. doi:. PMID 17253482.
- ^ Kaur B, Rowe BH, Arnold E (2009). "Vitamin C supplementation for asthma". Cochrane Database Syst Rev (1): CD000993. doi:. PMID 19160185.
- ^ Hemilä H, Koivula TT (2008). "Vitamin C for preventing and treating tetanus". Cochrane Database Syst Rev (2): CD006665. doi:. PMID 18425960.
- ^ High Doses of Vitamin C Are Not Effective as a Cancer Treatment
- ^ "FDA OKs vitamin C trial for cancer". Physorg.com. January 12, 2007. http://www.physorg.com/news87833644.html. Retrieved on 2007-04-06. "Federal approval of a clinical trial on intravenous vitamin C as a cancer treatment lends credence to alternative cancer care, U.S. researchers said."
- ^ Yeom CH, Jung GC, Song KJ (2007). "Changes of terminal cancer patients' health-related quality of life after high dose vitamin C administration". J. Korean Med. Sci. 22 (1): 7–11. PMID 17297243.
- ^ http://www.sciencedaily.com/releases/2008/08/080804190645.htm from Science News, Vitamin C Injections Slow Tumor Growth In Mice as reported in ScienceDaily Aug. 5, 2008, retrieved August 5 2008
- ^ "Vitamin C (Ascorbic acid)". MedLine Plus. National Institute of Health. 2006-08-01. http://www.nlm.nih.gov/medlineplus/druginfo/natural/patient-vitaminc.html. Retrieved on 2007-08-03.
- ^ Vitamin C by the American Cancer Society
- ^ "Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases". http://www.cochrane.org/reviews/en/ab007176.html. Retrieved on 2008-08-05.
- ^ Huang HY, Caballero B, Chang S, et al (May 2006). "Multivitamin/mineral supplements and prevention of chronic disease". Evid Rep Technol Assess (Full Rep) (139): 1–117. PMID 17764205. http://www.ahrq.gov/downloads/pub/evidence/pdf/multivit/multivit.pdf.
- ^ D (2008), "Antioxidant supplements for prevention of mortality in healthy participants and patients with …", Cochrane Database Syst Rev 2, doi:
- ^ Brzozowska A, Kaluza J, Knoops KT, de Groot LC (April 2008). "Supplement use and mortality: the SENECA study". Eur J Nutr 47 (3): 131–7. doi:. PMID 18414768.
- ^ Emadi-Konjin P, Verjee Z, Levin A, Adeli K (2005). "Measurement of intracellular vitamin C levels in human lymphocytes by reverse phase high performance liquid chromatography (HPLC)" (PDF). Clinical Biochemistry 38 (5): 450–6. doi:. PMID 15820776. http://www.sciencedirect.com/science?_ob=MImg&_imagekey=B6TDD-4FMHSY9-2-1&_cdi=5196&_user=308069&_orig=search&_coverDate=05%2F31%2F2005&_sk=999619994&view=c&wchp=dGLzVzz-zSkWW&md5=05a80321d3767c0cdfd386ef7121a781&ie=/sdarticle.pdf.
- ^ Yamada H, Yamada K, Waki M, Umegaki K. (2004). "Lymphocyte and Plasma Vitamin C Levels in Type 2 Diabetic Patients With and Without Diabetes Complications" (PDF). Diabetes Care 27: 2491–2. doi:. PMID 15451922. http://care.diabetesjournals.org/cgi/reprint/27/10/2491.pdf.
- ^ "Toxicological evaluation of some food additives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents". World Health Organization. 4 July 1973. http://www.inchem.org/documents/jecfa/jecmono/v05je20.htm. Retrieved on 2007-04-13.
- ^ Fleming DJ, Tucker KL, Jacques PF, Dallal GE, Wilson PW, Wood RJ (2002). "Dietary factors associated with the risk of high iron stores in the elderly Framingham Heart Study cohort" (PDF). Am. J. Clin. Nutr. 76 (6): 1375–84. PMID 12450906. http://www.ajcn.org/cgi/reprint/76/6/1375.pdf.
- ^ Cook JD, Reddy MB (2001). "Effect of ascorbic acid intake on nonheme-iron absorption from a complete diet" (PDF). Am. J. Clin. Nutr. 73 (1): 93–8. PMID 11124756. http://www.ajcn.org/cgi/reprint/73/1/93.pdf.
- ^ Goodwin JS, Tangum MR (November 1998). "Battling quackery: attitudes about micronutrient supplements in American academic medicine". Arch. Intern. Med. 158 (20): 2187–91. doi:. PMID 9818798. http://archinte.ama-assn.org/cgi/pmidlookup?view=long&pmid=9818798.
- ^ Massey LK, Liebman M, Kynast-Gales SA (2005). "Ascorbate increases human oxaluria and kidney stone risk" (PDF). J. Nutr. 135 (7): 1673–7. PMID 15987848. http://jn.nutrition.org/cgi/reprint/135/7/1673.pdf.
- ^ Naidu KA (2003). "Vitamin C in human health and disease is still a mystery? An overview" (PDF). J. Nutr. 2 (7): 7. doi:. PMID 14498993. http://www.nutritionj.com/content/pdf/1475-2891-2-7.pdf.
- ^ Ovcharov R, Todorov S (1974). "[The effect of vitamin C on the estrus cycle and embryogenesis of rats]" (in Bulgarian). Akusherstvo i ginekologii͡a 13 (3): 191–5. PMID 4467736.
- ^ Vobecky JS, Vobecky J, Shapcott D, Cloutier D, Lafond R, Blanchard R (1976). "Vitamins C and E in spontaneous abortion". International journal for vitamin and nutrition research. Internationale Zeitschrift für Vitamin- und Ernährungsforschung. Journal international de vitaminologie et de nutrition 46 (3): 291–6. PMID 988001.
- ^ Javert CT, Stander HJ (1943). "Plasma Vitamin C and Prothrombin Concentration in Pregnancy and in Threatened, Spontaneous, and Habitual Abortion". Surgery, Gynecology, and Obstetrics 76: 115–122.
- ^ Mari-Carmen Gomez-Cabrera et al. (2008-01). "Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance". American Journal of Clinical Nutrition 87 (1): 142-9. PMID 18175748.
- ^ Wilson JX (2005). "Regulation of vitamin C transport". Annu. Rev. Nutr. 25: 105–25. doi:. PMID 16011461.
- ^ "The vitamin and mineral content is stable". Danish Veterinary and Food Administration. http://www.uk.foedevarestyrelsen.dk/Nutrition/Vitamin_mineral_content_is_stable/forside.htm. Retrieved on 2007-03-07.
- ^ "National Nutrient Database". Nutrient Data Laboratory of the US Agricultural Research Service. http://www.nal.usda.gov/fnic/foodcomp/search/. Retrieved on 2007-03-07.
- ^ "Vitamin C Food Data Chart". Healthy Eating Club. http://www.healthyeatingclub.com/info/books-phds/books/foodfacts/html/data/data4i.html. Retrieved on 2007-03-07.
- ^ "Natural food-Fruit Vitamin C Content". The Natural Food Hub. http://www.naturalhub.com/natural_food_guide_fruit_vitamin_c.htm. Retrieved on 2007-03-07.
- ^ Clark, Stephanie, Ph. D (8 January 2007). "Comparing Milk: Human, Cow, Goat & Commercial Infant Formula". Washington State University. http://www.saanendoah.com/compare.html. Retrieved on 2007-02-28.
- ^ Toutain, P. L.; D. Béchu, and M. Hidiroglou (November 1997). "Ascorbic acid disposition kinetics in the plasma and tissues of calves". Am J Physiol Regul Integr Comp Physiol Vol. 273, (Issue 5, R1585-R1597). http://ajpregu.physiology.org/cgi/content/full/273/5/R1585.
- ^ Combs GF. The Vitamins, Fundamental Aspects in Nutrition and Health. 2nd ed. San Diego, CA: Academic Press, 2001:245–272
- ^ Hitti, Miranda (2 June 2006). "Fresh-Cut Fruit May Keep Its Vitamins". WebMD. http://www.webmd.com/content/article/123/115022.htm. Retrieved on 2007-02-25.
- ^ The Diet Channel Vitamin C might be the most widely known and most popular vitamin purchased as a supplement.
- ^ "The production of vitamin C" (PDF). Competition Commission. 2001. http://www.competition-commission.org.uk/rep_pub/reports/2001/fulltext/456a4.2.pdf. Retrieved on 2007-02-20.
- ^ Patton, Dominique (2005-10-20). "DSM makes last stand against Chinese vitamin C". nutraingredients. http://www.nutraingredients.com/news/ng.asp?n=63349-dsm-vitamin-c. Retrieved on 2007-02-20.
- ^ DSM vitamin plant gains green thumbs-up Shane Starling,Decision News Media SAS , 26-Jun-2008. Accessed July 2008
- ^ Vitamin C: Distruptions to Production in China to Maintain Firm Market FLEXNEWS, 30/06/2008, Accessed July 2008
- ^ [2], Addition of Vitamins and Minerals to Food, 2005: Health Canada's Proposed Policy and Implementation Plans.
[edit] Further reading
- Books
- Pauling, Linus (1976). Vitamin C, the Common Cold, and the Flu. W H Freeman & Co. ISBN 0716703610. OCLC 2388395.
- Cameron, Ewan; Linus Pauling, (1979). Cancer and Vitamin C. Pauling Institute of Science and Medicine. ISBN 0393500004. OCLC 5788147.
- Clemetson, C.A.B (1989). Vitamin C. Boca Raton, Florida: CRC Press. ISBN 0-8493-4841-2. OCLC 165609070 17918592. Monograph - Volumes I, II, III.
[edit] External links
- Jane Higdon, "Vitamin C", Micronutrient Information Center, Linus Pauling Institute
- U.S. Patent 5,278,189 — "Prevention and treatment of occlusive cardiovascular disease with ascorbate and substances that inhibit the binding of lipoprotein (a)", Inventors: Matthias W. Rath and Linus C. Pauling
- vitamin C at United Kingdom Food Standards Agency
- Naidu KA (2003). "Vitamin C in human health and disease is still a mystery? An overview". Nutrition journal 2: 7. doi:. PMID 14498993. http://www.nutritionj.com/content/2/1/7.
- Vitamin C Requirements: Optimal Health Benefits vs Overdose — a moderate dose advocacy site
- Vitamin C information from U.S. MedlinePlus Health Information
|
|||||||||||||||||||||||||||