Paracetamol
From Wikipedia, the free encyclopedia
 |
|
 |
|
|
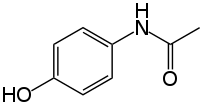
Paracetamol
|
|
| Systematic (IUPAC) name | |
| N-(4-hydroxyphenyl)acetamide | |
| Identifiers | |
| CAS number | |
| ATC code | N02 |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| Chemical data | |
| Formula | C8H9NO2 |
| Mol. mass | 151.169 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | almost 100% |
| Metabolism | 90 to 95% Hepatic |
| Half life | 1–4 h |
| Excretion | Renal |
| Therapeutic considerations | |
| Licence data | |
| Pregnancy cat. | |
| Legal status | |
| Routes | Oral, rectal, intravenous |
Paracetamol (INN) (pronounced /ˌpærəˈsiːtəmɒl, -ˈsɛtə-/) or acetaminophen (USAN) is a widely used over-the-counter analgesic (pain reliever) and antipyretic (fever reducer). It is commonly used for the relief of fever, headaches, and other minor aches and pains, and is a major ingredient in numerous cold and flu remedies. In combination with non-steroidal anti-inflammatory drugs (NSAIDs) or opioid analgesics, paracetamol is used also in the management of more severe pain (such as cancer pain).[1]
While generally safe for human use at recommended doses, acute overdoses of paracetamol can cause potentially fatal liver damage and, in rare individuals, a normal dose can do the same; the risk is heightened by alcoholism. Paracetamol toxicity is the foremost cause of acute liver failure in the Western world, and accounts for most drug overdoses in the United States, the United Kingdom, Australia and New Zealand.[2][3][4][5] In 2008 a study conducted in 31 countries on over 200,000 children indicates that infants who are given paracetamol may be at an increased risk of developing asthma as children.[6]
Paracetamol is derived from coal tar, and is therefore part of the class of drugs known as “aniline analgesics”; it is the only such drug still in use today.[7] It is the active metabolite of phenacetin, once popular as an analgesic and antipyretic in its own right, but unlike phenacetin and its combinations, paracetamol is not considered carcinogenic at therapeutic doses.[8] The words acetaminophen and paracetamol both come from chemical names for the compound: para-acetylaminophenol and para-acetylaminophenol. In some contexts, it is simply abbreviated as APAP, for N-acetyl-para-aminophenol.
Contents |
[edit] History
Acetanilide was the first aniline derivative serendipitously found to possess analgesic as well as antipyretic properties, and was quickly introduced into medical practice under the name of Antifebrin by A. Cahn and P. Hepp in 1886.[9] But its unacceptable toxic effects, the most alarming being cyanosis due to methemoglobinemia, prompted the search for less toxic aniline derivatives.[7] Harmon Northrop Morse had already synthesized paracetamol at John Hopkins University via the reduction of p-nitrophenol with tin in glacial acetic acid in 1877,[10][11] but it wasn't until 1887 that clinical pharmacologist Joseph von Mering tried paracetamol on patients.[7] In 1893 von Mering published a paper reporting on the clinical results of paracetamol with phenacetin, another aniline derivative.[12] Von Mering claimed that, unlike phenacetin, paracetamol had a slight tendency to produce methemoglobinemia. Paracetamol was then quickly discarded in favor of phenacetin. The sales of phenacetin established Bayer as a leading pharmaceutical company.[13] Overshadowed in part by aspirin, introduced into medicine by Heinrich Dreser in 1899, phenacetin was popular for many decades, particularly in widely advertised over-the-counter “headache mixtures,” usually containing phenacetin, an aminopyrine derivative or aspirin, caffeine, and sometimes a barbiturate.[7]
Von Mering's claims remained essentially unchallenged for half a century, until two teams of researchers from the United States analyzed the metabolism of acetanilide and paracetamol.[13] In 1947 David Lester and Leon Greenberg found strong evidence that paracetamol was a major metabolite of acetanilide in human blood,[14] and in a subsequent study they reported that large doses of paracetamol given to albino rats did not cause methemoglobinemia.[15] In three papers published in the September 1948 issue of the Journal of Pharmacology and Experimental Therapeutics, Bernard Brodie, Julius Axelrod and Frederick Flinn confirmed using more specific methods that paracetamol was the major metabolite of acetanilide in human blood, and established it was a just as efficacious analgesic as its precursor.[16][17][18] They also suggested that methemoglobinemia is produced in humans mainly by another metabolite, phenylhydroxylamine. A followup paper by Brodie and Axelrod in 1949 established that phenacetin was also metabolized to paracetamol.[19] This led to a "rediscovery" of paracetamol.[7] It has been suggested that contamination of paracetamol with 4-aminophenol, the substance from which it was sythesized by von Mering, may be the cause for his spurious findings.[13]

Paracetamol was first marketed in the United States in 1953 by Sterling-Winthrop Co., which promoted it as preferable to aspirin since it was safe to take for children and people with ulcers.[13] The best known brand today for paracetamol in the United States, Tylenol, was established in 1955 when McNeil Laboratories started selling paracetamol as a pain and fever reliever for children, under the brand name Tylenol Children's Elixir—the word "tylenol" was a contraction of para-acetylaminophenol.[20] In 1956, 500 mg tablets of paracetamol went on sale in the United Kingdom under the trade name Panadol, produced by Frederick Stearns & Co, a subsidiary of Sterling Drug Inc. Panadol was originally available only by prescription, for the relief of pain and fever, and was advertised as being "gentle to the stomach," since other analgesic agents of the time contained aspirin, a known stomach irritant.[citation needed] In 1963, paracetamol was added to the British Pharmacopoeia, and has gained popularity since then as an analgesic agent with few side-effects and little interaction with other pharmaceutical agents.[11] Concerns about paracetamol's safety delayed its widespread acceptance until the 1970s, but in the 1980s paracetamol sales exceeded those of aspirin in many countries, including the United Kingdom. This was accompanied by the commercial demise of phenacetin, blamed as the cause of analgesic nephropathy, hematological toxicity, and liability for abuse due to psychotropic effects.[7]
The U.S. patent on paracetamol has long expired, and generic versions of the drug are widely available under the Drug Price Competition and Patent Term Restoration Act of 1984, although certain Tylenol preparations were protected until 2007. U.S. patent 6,126,967 filed September 3, 1998 was granted for "Extended release acetaminophen particles".[21]
[edit] Structure and reactivity

Paracetamol consists of a benzene ring core, substituted by one hydroxyl group and the nitrogen atom of an amide group in the para (1,4) pattern.[22] The amide group is acetamide (ethanamide). It is an extensively conjugated system, as the lone pair on the hydroxyl oxygen, the benzene pi cloud, the nitrogen lone pair, the p orbital on the carbonyl carbon, and the lone pair on the carbonyl oxygen are all conjugated. The presence of two activating groups also make the benzene ring highly reactive toward electrophilic aromatic substitution. As the substituents are ortho,para-directing and para with respect to each other, all positions on the ring are more or less equally activated. The conjugation also greatly reduces the basicity of the oxygens and the nitrogen, while making the hydroxyl acidic through delocalisation of charge developed on the phenoxide anion.
[edit] Synthesis
Compared with many other drugs, paracetamol is much easier to synthesize, because it lacks stereocenters. As a result, there is no need to design a stereo-selective synthesis.
Industrial preparation of paracetamol usually proceeds from nitrobenzene.[23] A one-step reductive acetamidation reaction can be mediated by thioacetate.[24]
Paracetamol may be easily prepared in the laboratory by nitrating phenol with sodium nitrate, separating the desired p-nitrophenol from the ortho- byproduct, and reducing the nitro group with sodium borohydride. The resultant p-aminophenol is then acetylated with acetic anhydride.[25] In this reaction, phenol is strongly activating, thus the reaction only requires mild conditions (c.f. the nitration of benzene):
[edit] Reactions
p-Aminophenol may be obtained by the amide hydrolysis of paracetamol. p-Aminophenol prepared this way, and related to the commercially available Metol, has been used as a developer in photography by hobbyists.[26]
[edit] Available forms
| This section may require copy-editing for rambling. You can assist by editing it now. A how-to guide is available. (January 2009) |
| This section does not cite any references or sources. Please help improve this article by adding citations to reliable sources (ideally, using inline citations). Unsourced material may be challenged and removed. (January 2009) |


Panadol, which is marketed in Africa, Asia, Central America, and Australasia, is the most widely available brand, sold in over 80 countries. In North America, paracetamol is sold in generic form (usually labeled as acetaminophen) or under a number of trade names, for instance, Tylenol (McNeil-PPC, Inc.), Anacin-3, Tempra, and Datril. While there is brand named paracetamol available in the UK (e.g. Panadol), unbranded or generic paracetamol is more commonly sold. In Europe, the most common brands of paracetamol are Efferalgan and Doliprane.
In some formulations, paracetamol is combined with the opioid codeine, sometimes referred to as co-codamol (BAN). In the United States and Canada, this is marketed under the name of Tylenol #1/2/3/4, which contain 8-10 mg, 15 mg, 30 mg, and 60 mg of codeine, respectively. In the U.S., this combination is available only by prescription, while the lowest-strength preparation is over-the-counter in Canada, and, in other countries, other strengths may be available over the counter. There are generic forms of these combinations as well. In the UK and in many other countries, this combination is marketed under the names of Tylex CD and Panadeine. Other names include Captin, Disprol, Dymadon, Fensum, Hedex, Mexalen, Nofedol, Paralen, Pediapirin, Perfalgan, and Solpadeine. Paracetamol is also combined with other opioids such as dihydrocodeine, referred to as co-dydramol (BAN), oxycodone or hydrocodone, marketed in the U.S. as Percocet and Vicodin, respectively. Another very commonly used analgesic combination includes paracetamol in combination with propoxyphene napsylate, sold under the brand name Darvocet. A combination of paracetamol, codeine, and the calmative doxylamine succinate is marketed as Syndol or Mersyndol.
Paracetamol is commonly used in multi-ingredient preparations for migraine headache, typically including butalbital and paracetamol with or without caffeine, and sometimes containing codeine.
It is commonly administered in tablet, liquid suspension, suppository, intravenous, or intramuscular form. The common adult dose is 500 mg to 1000 mg. The recommended maximum daily dose, for adults, is 4 grams. In recommended doses, paracetamol generally is safe for children and infants, as well as for adults.
| Brand Names[27] |
|---|
| Aceta, Actimin, Anacin-3, Apacet, Aspirin Free Anacin, Atasol, Banesin, Crocin, Dapa, Datril Extra-Strength, DayQuil, Depon & Depon Maximum, Feverall, Few Drops, Fibi, Fibi plus, Genapap, Genebs, Lekadol, Liquiprin, Neopap, Oraphen-PD, Panadol, Paralen, Phenaphen, Redutemp, Snaplets-FR, Suppap, Tapanol, Tempra, Tylenol, Valorin, Xcel. |
[edit] Mechanism of action
Paracetamol is usually classified along with nonsteroidal antiinflammatory drugs (NSAID). Like all drugs of this class, its main mechanism of action is the inhibition of cyclooxygenase (COX), an enzyme responsible for the production of prostaglandins, which are important mediators of inflammation, pain and fever. Therefore, all NSAIDS are said to possess antiinflammatory, analgesic (anti-pain), and antipyretic (anti-fever) properties. The specific actions of each NSAID drug depends upon their pharmacological properties, distribution and metabolism.
While paracetamol has analgesic and antipyretic properties comparable to those of aspirin, it fails to exert significant antiinflammatory action due to paracetamol's susceptibility to the high level of peroxides present in inflammatory lesions.

However, the mechanism by which paracetamol reduces fever and pain is still debated[28] largely because paracetamol reduces the production of prostaglandins (pro-inflammatory chemicals). Aspirin also inhibits the production of prostaglandins, but, unlike aspirin, paracetamol has little anti-inflammatory action. Likewise, whereas aspirin inhibits the production of the pro-clotting chemicals thromboxanes, paracetamol does not. Aspirin is known to inhibit the cyclooxygenase (COX) family of enzymes, and, because of paracetamol's partial similarity of aspirin's action, much research has focused on whether paracetamol also inhibits COX. It is now clear that paracetamol acts via at least two pathways.[29][30][7][31]
The COX family of enzymes are responsible for the metabolism of arachidonic acid to prostaglandin H2, an unstable molecule, which is, in turn, converted to numerous other pro-inflammatory compounds. Classical anti-inflammatories, such as the NSAIDs, block this step. Only when appropriately oxidized is the COX enzyme highly active.[32][33] Paracetamol reduces the oxidized form of the COX enzyme, preventing it from forming pro-inflammatory chemicals.[30][34]
Paracetamol also modulates the endogenous cannabinoid system.[35] Paracetamol is metabolized to AM404, a compound with several actions; most important, it inhibits the uptake of the endogenous cannabinoid/vanilloid anandamide by neurons. Anandamide uptake would result in the activation of the main pain receptor (nociceptor) of the body, the TRPV1 (older name: vanilloid receptor). Furthermore, AM404 inhibits sodium channels, as do the anesthetics lidocaine and procaine.[36] Either of these actions by themselves has been shown to reduce pain, and are a possible mechanism for paracetamol, though it has been demonstrated that, after blocking cannabinoid receptors and hence making any action of cannabinoid reuptake irrelevant, paracetamol loses analgesic effect, suggesting its pain-relieving action is mediated by the endogenous cannabinoid system.[37]
One theory holds that paracetamol works by inhibiting the COX-3 isoform of the COX family of enzymes. This enzyme, when expressed in dogs, shares a strong similarity to the other COX enzymes, produces pro-inflammatory chemicals, and is selectively inhibited by paracetamol.[38] However, some research has suggested that in humans and mice, the COX-3 enzyme is without inflammatory action.[29] Another possibility is that paracetamol blocks cyclooxygenase (as in aspirin), but that in an inflammatory environment, where the concentration of peroxides is high, the oxidation state of paracetamol is high which prevents its actions. This would mean that paracetamol has no direct effect at the site of inflammation but instead acts in the CNS to reduce temperature etc where the environment is not oxidative.[38] The exact mechanism by which paracetamol is believed to affect COX-3 is disputed.
[edit] Metabolism

Paracetamol is metabolised primarily in the liver, into non-toxic products. Three metabolic pathways are notable:
- Glucuronidation is believed to account for 40% to two-thirds of the metabolism of paracetamol.[39]
- Sulfation (sulfate conjugation) may account for 20–40%.[39]
- N-hydroxylation and rearrangement, then GSH conjugation, accounts for less than 15%. The hepatic cytochrome P450 enzyme system metabolizes paracetamol, forming a minor yet significant alkylating metabolite known as NAPQI (N-acetyl-p-benzo-quinone imine).[40] NAPQI is then irreversibly conjugated with the sulfhydryl groups of glutathione.[40]
All three pathways yield final products that are inactive, non-toxic, and eventually excreted by the kidneys. In the third pathway, however, the intermediate product NAPQI is toxic. NAPQI is primarily responsible for the toxic effects of paracetamol; this constitutes an excellent example of toxication.
Production of NAPQI is due primarily to two isoenzymes of cytochrome P450: CYP2E1 and CYP1A2. The P450 gene is highly polymorphic, however, and individual differences in paracetamol toxicity are believed to be due to a third isoenzyme, CYP2D6. Genetic polymorphisms in CYP2D6 may contribute to significantly different rates of production of NAPQI. Furthermore, individuals can be classified as "extensive", "ultrarapid", and "poor" metabolizers (producers of NAPQI), depending on their levels of CYP2D6 expression. Although CYP2D6 metabolises paracetamol into NAPQI to a lesser extent than other P450 enzymes, its activity may contribute to paracetamol toxicity in extensive and ultrarapid metabolisers, and when paracetamol is taken at very large doses.[41] At usual doses, NAPQI is quickly detoxified by conjugation.[40] Following overdose, and possibly also in extensive and ultrarapid metabolizers, this detoxification pathway becomes saturated and consequently NAPQI accumulates.
[edit] Indications
| Please help improve this article or section by expanding it. Further information might be found on the talk page. (January 2009) |
The WHO recommends that paracetamol be given to children with fever higher than 38.5°C. (101.3 °F)[42]
Paracetamol is a suitable substitute for aspirin, especially in patients where excessive gastric acid secretion or prolongation of bleeding time may be a concern. While paracetamol has analgesic and antipyretic properties comparable to those of aspirin, its anti-inflammatory effects are weak. Because acetaminophen is well tolerated, available without a prescription, and lacks the gastric side effects of aspirin, it has in recent years increasingly become a common household drug.
[edit] Efficacy and side effects
Paracetamol, unlike other common analgesics such as aspirin and ibuprofen, has relatively little anti-inflammatory activity, so it is not considered to be a non-steroidal anti-inflammatory drug (NSAID).
[edit] Efficacy
Regarding comparative efficacy, studies show conflicting results when compared to NSAIDs. A randomized controlled trial of chronic pain from osteoarthritis in adults found similar benefit from paracetamol and ibuprofen.[43][unreliable source?][44] However, a randomized controlled trial of acute musculoskeletal pain in children found that the standard OTC dose of ibuprofen gives greater relief of pain than the standard dose of paracetamol.[45][unreliable source?]
[edit] Adverse effects
In recommended doses, paracetamol does not irritate the lining of the stomach, affect blood coagulation as much as NSAIDs, or affect function of the kidneys.[citation needed] However, some studies have shown that high dose-usage (greater than 2,000 mg per day) does increase the risk of upper gastrointestinal complications such as stomach bleeding.[46] Paracetamol is safe in pregnancy, and does not affect the closure of the fetal ductus arteriosus as NSAIDs can.[47] Unlike aspirin, it is safe in children, as paracetamol is not associated with a risk of Reye's syndrome in children with viral illnesses.[48]
Like NSAIDs and unlike opioid analgesics, paracetamol has not been found to cause euphoria or alter mood in any way. While paracetamol and NSAIDs may damage the liver, they do not pose a large risk of addiction, dependence, tolerance, and withdrawal.[citation needed] Paracetamol, particularly in combination with weak opioids, is more likely than NSAIDs to cause rebound headache (medication overuse headache), although less of a risk than ergotamine or triptans used for migraines.[49][unreliable source?]
In 2008 the Lancet published the largest study to date on long term side effects of paracetamol in children. Conducted on over 200,000 children in 31 countries, the study determined that use of paracetamol for fever in the first year of life was associated with a 46% increase in the risk of developing asthma symptoms when aged 6–7 years. Higher doses and more regular use of the drug are associated with a greater risk of developing asthma; up to a three-fold increase for heavy use. Furthermore, paracetamol use, both in the first year of life and in children aged 6–7 years, was associated with an increased risk of symptoms of rhino conjunctivitis and eczema.[6] In that article the authors acknowledged that "our findings might have been due to confounding by indication", i.e. that the association they found was not causal but rather due to the disease being treated with paracetamol, and emphasized that further research is needed.[6]
[edit] Toxicity
Excessive use of paracetamol can damage multiple organs, especially the liver and kidney. In both organs, toxicity from paracetamol is not from the drug itself but from one of its metabolites, N-acetyl-p-benzoquinoneimine (NAPQI). In the liver, the cytochrome P450 enzymes CYP2E1 and CYP3A4 are primarily responsible for the conversion of paracetamol to NAPQI. In the kidney, cyclooxygenases are the principal route by which paracetamol is converted to NAPQI.[50] Paracetamol overdose leads to the accumulation of NAPQI, which undergoes conjugation with glutathione. Conjugation depletes glutathione, a natural antioxidant. This in combination with direct cellular injury by NAPQI, leads to cell damage and death.[51]
Paracetamol hepatotoxicity is, by far, the most common cause of acute liver failure in both the United States and the United Kingdom.[5][52] Paracetamol overdose results in more calls to poison control centers in the US than overdose of any other pharmacological substance.[53] Signs and symptoms of paracetamol toxicity may initially be absent or vague. Untreated, overdose can lead to liver failure and death within days. Treatment is aimed at removing the paracetamol from the body and replacing glutathione. Activated charcoal can be used to decrease absorption of paracetamol if the patient presents for treatment soon after the overdose. While the antidote, acetylcysteine, acts as a precursor for glutathione helping the body regenerate enough to prevent damage to the liver, a liver transplant is often required if damage to the liver becomes severe.[2]
[edit] Effects on animals
Paracetamol is extremely toxic to cats, and should not be given to them under any circumstances. Cats lack the necessary glucuronyl transferase enzymes to safely break paracetamol down, and minute portions of a tablet may prove fatal. Initial symptoms include vomiting, salivation and discolouration of the tongue and gums. Unlike an overdose in humans, liver damage is rarely the cause of death; instead, methaemoglobin formation and the production of Heinz bodies in red blood cells inhibit oxygen transport by the blood, causing asphyxiation (methemoglobemia and hemolytic anemia).[54] Treatment with N-acetylcysteine, methylene blue or both is sometimes effective after the ingestion of small doses of paracetamol. Female cats have a better survival rate.[55]
Although paracetamol is believed to have no significant anti-inflammatory activity, it has been reported to be as effective as aspirin in the treatment of musculoskeletal pain in dogs.[56] A paracetamol-codeine product (trade name Pardale-V)[57] licensed for use in dogs is available on veterinary prescription in the UK.[58] It should be administered to dogs only on veterinary advice. The main effects of toxicity in dogs is liver damage.[59] N-acetylcysteine treatment is efficacious in dogs when administered within a few hours of paracetamol ingestion.[56]
Paracetamol is also lethal to snakes, and has been suggested as chemical control program for the brown tree snake (Boiga irregularis) in Guam.[60]
[edit] Notes and references
- ^ Control of Pain in Patients with Cancer Sign Guidelines 40 Section 6 [1].
- ^ a b Daly FF, Fountain JS, Murray L, Graudins A, Buckley NA (March 2008). "Guidelines for the management of paracetamol poisoning in Australia and New Zealand—explanation and elaboration. A consensus statement from clinical toxicologists consulting to the Australasian poisons information centres". Med. J. Aust. 188 (5): 296–301. PMID 18312195. http://www.mja.com.au/public/issues/188_05_030308/dal10916_fm.html.
- ^ Khashab M, Tector AJ, Kwo PY (March 2007). "Epidemiology of acute liver failure". Curr Gastroenterol Rep 9 (1): 66–73. PMID 17335680.
- ^ Hawkins LC, Edwards JN, Dargan PI (2007). "Impact of restricting paracetamol pack sizes on paracetamol poisoning in the United Kingdom: a review of the literature". Drug Saf 30 (6): 465–79. PMID 17536874.
- ^ a b Larson AM, Polson J, Fontana RJ, et al (2005). "Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study". Hepatology 42 (6): 1364–72. doi:. PMID 16317692.
- ^ a b c Beasley, Richard; Clayton, Tadd; Crane, Julian; von Mutius, Erika; Lai, Christopher; Montefort, Stephen; Stewart, Alistair (2008). "Association between paracetamol use in infancy and childhood, and risk of asthma, rhino conjunctivitis, and eczema in children aged 6–7 years: analysis from Phase Three of the ISAAC programme.". The Lancet 372: 1039–1048. http://www.thelancet.com/journals/lancet/article/PIIS0140673608614452/abstract. Retrieved on 2008-09-19. Lay summary – The Independent (September 19, 2008).
- ^ a b c d e f g Bertolini A, Ferrari A, Ottani A, Guerzoni S, Tacchi R, Leone S (2006). "Paracetamol: new vistas of an old drug". CNS drug reviews 12 (3–4): 250–75. doi:. PMID 17227290.
- ^ Bergman K, Müller L, Teigen SW (February 1996). "The genotoxicity and carcinogenicity of paracetamol: a regulatory (re)view". Mutat Res 349 (2): 263–88. doi:. PMID 8600357.
- ^ Chan A, Hepp P. Das Antifebrin, ein neues Fiebermittel. Centralbl Klein Med 1886;7:561–564.
- ^ H. N. Morse (1878). "Ueber eine neue Darstellungsmethode der Acetylamidophenole". Berichte der deutschen chemischen Gesellschaft 11 (1): 232–233. doi:.
- ^ a b Milton Silverman, Mia Lydecker, Philip Randolph Lee (1992). Bad Medicine: The Prescription Drug Industry in the Third World. Stanford University Press. pp. 88–90. ISBN 0804716692.
- ^ Von Mering J. Beitrage zur Kenntniss der Antipyretica. Ther Monatsch 1893;7:577–587.
- ^ a b c d Sneader, Walter (2005). Drug Discovery: A History. p. 439. ISBN 0471899801.
- ^ D. Lester, L.A. Greenberg, Metabolic fate of acetanilide and other aniline derivatives. II. Major metabolites of acetanilide in the blood., J. Pharm. Exp. Ther., 1947; 90: 68
- ^ D. Lester, L.A. Greenberg, Metabolic fate of acetanilide and other aniline derivatives. II. Major metabolites of acetanilide in the blood., J. Pharm. Exp. Ther., 1947; 90: 68
- ^ Brodie BB, Axelrod J (1948). "The estimation of acetanilide and its metabolic products, aniline, N-acetyl p-aminophenol and p-aminophenol (free and total conjugated) in biological fluids and tissues". J Pharmacol Exp Ther 94 (1): 22–28.
- ^ Brodie BB, Axelrod J (1948). "The fate of acetanilide in man" (PDF). J Pharmacol Exp Ther 94 (1): 29–38. http://profiles.nlm.nih.gov/HH/A/A/A/D/_/hhaaad.pdf.
- ^ Frederick B. Flinn, Bernard B. Brodie (1948). "The effect on the pain threshold of N-acetyl p-aminophenol, a product derived in the body from acetanilide". J Pharmacol Exp Ther 94 (1): 76–77.
- ^ Brodie BB, Axelrod J (1949). "The fate of acetophenetidin (phenacetin) in man and methods for the estimation of acetophenitidin and its metabolites in biological material". J Pharmacol Exp Ther 94 (1): 58-67.
- ^ "A Festival of Analgesics." Chemical Heritage Foundation. 2001. Retrieved on August 17, 2007.
- ^ US patent 6126967, "Extended release acetaminophen particles", granted 2000-10-03
- ^ Bales, JR; Nicholson JK, Sadler PJ (May 1985). "Two-dimensional proton nuclear magnetic resonance "maps" of acetaminophen metabolites in human urine". Clinical Chemistry 31 (5): 757–762. PMID 3987005. http://www.clinchem.org/cgi/reprint/31/5/757.
- ^ Anthony S. Travis (2007). "Manufacture and uses of the anilines: A vast array of processes and products". in Zvi Rappoport. The chemistry of Anilines Part 1. Wiley. pp. 764. ISBN 978-0-470-87171-3.
- ^ Bhattacharya A.; Purohit V. C.; Suarez, V.; Tichkule, R; Parmer, G.; Rinaldi, F. (2006). "One-step reductive amidation of nitro arenes: application in the synthesis of Acetaminophen". Tetrahedron Letters 47 (11): 1861–1864. doi:.
- ^ Ellis, Frank (2002). Paracetamol: a curriculum resource. Cambridge: Royal Society of Chemistry. ISBN 0-85404-375-6.
- ^ Henney, K; Dudley B (1939). Handbook of Photography. Whittlesey House. pp. 324.
- ^ Reader's Digest Guide to Drugs and Supplements. Pleasantville, New York; Montreal: Reader's Digest Association, Inc.. 2002. ISBN 0-7621-0366-3.
- ^ Rossi, S. (ed.) (2008). Australian Medicines Handbook 2008. Adelaide: Australian Medicines Handbook. ISBN 0-9757919-6-7. http://www.amh.net.au.
- ^ a b Kis B, Snipes JA, Busija DW (2005). "Acetaminophen and the cyclooxygenase-3 puzzle: sorting out facts, fictions, and uncertainties". J. Pharmacol. Exp. Ther. 315 (1): 1–7. doi:. PMID 15879007.
- ^ a b Aronoff DM, Oates JA, Boutaud O (2006). "New insights into the mechanism of action of acetaminophen: Its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin H2 synthases". Clin. Pharmacol. Ther. 79 (1): 9–19. doi:. PMID 16413237.
- ^ Graham GG, Scott KF (2005). "Mechanism of action of paracetamol". American journal of therapeutics 12 (1): 46–55. doi:. PMID 15662292.
- ^ Ohki S, Ogino N, Yamamoto S, Hayaishi O (1979). "Prostaglandin hydroperoxidase, an integral part of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes". J. Biol. Chem. 254 (3): 829–36. PMID 104998.
- ^ Harvison PJ, Egan RW, Gale PH, Nelson SD (1986). "Acetaminophen as a cosubstrate and inhibitor of prostaglandin H synthase". Adv. Exp. Med. Biol. 197: 739–47. PMID 3094341.
- ^ Roberts, L.J II. & Marrow, J.D. "Analgesic-antipyretic and Antiinflammatory Agents and Drugs Employed in the Treatment of Gout" in, "Goodman & Gilman's The Pharmacological Basis of Therapeutics 10th Edition" by Hardman, J.G. & Limbird, L.E. Published by McGraw Hill, 2001, p.687–731.
- ^ Högestätt ED, Jönsson BA, Ermund A, et al (2005). "Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system". J. Biol. Chem. 280 (36): 31405–12. doi:. PMID 15987694.
- ^ Köfalvi A (2008). Chapter 9: Alternative interacting sites and novel receptors for cannabinoid ligands. In: 'Cannabinoids and the Brain' Springer-Verlag. pp. 131–160. doi:.
- ^ Ottani A, Leone S, Sandrini M, Ferrari A, Bertolini A (2006). "The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors". Eur. J. Pharmacol. 531 (1–3): 280–1. doi:. PMID 16438952.
- ^ a b Chandrasekharan NV, Dai H, Roos KL, et al (2002). "COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression". Proc. Natl. Acad. Sci. U.S.A. 99 (21): 13926–31. doi:. PMID 12242329.
- ^ a b Hendrickson, Robert G.; Kenneth E. Bizovi (2006). “Acetaminophen”, in Nelson, Lewis H.; Flomenbaum, Neal; Goldfrank, Lewis R. et al. Goldfrank's toxicologic emergencies, p. 525, New York: McGraw-Hill. Retrieved on January 18, 2009 through Google Book Search.
- ^ a b c Borne, Ronald F. "Nonsteroidal Anti-inflammatory Drugs" in Principles of Medicinal Chemistry, Fourth Edition. Eds. Foye, William O.; Lemke, Thomas L.; Williams, David A. Published by Williams & Wilkins, 1995. p. 544–545.
- ^ Dong H, Haining RL, Thummel KE, Rettie AE, Nelson SD (2000). "Involvement of human cytochrome P450 2D6 in the bioactivation of acetaminophen". Drug Metab Dispos 28 (12): 1397–400. PMID 11095574. Free full text
- ^ "Baby paracetamol asthma concern". BBC News. 2008-09-19. http://news.bbc.co.uk/1/hi/health/7623230.stm. Retrieved on 2008-09-19.
- ^ Bradley JD, Brandt KD, Katz BP, Kalasinski LA, Ryan SI (1991). "Comparison of an antiinflammatory dose of ibuprofen, an analgesic dose of ibuprofen, and acetaminophen in the treatment of patients with osteoarthritis of the knee". N. Engl. J. Med. 325 (2): 87–91. PMID 2052056.
- ^ doi:10.1111/j.1365-2710.2006.00754.x
- ^ Clark E, Plint AC, Correll R, Gaboury I, Passi B (2007). "A randomized, controlled trial of acetaminophen, ibuprofen, and codeine for acute pain relief in children with musculoskeletal trauma". Pediatrics 119 (3): 460–7. doi:. PMID 17332198.
- ^ García Rodríguez LA, Hernández-Díaz S (December 15, 2000). "The risk of upper gastrointestinal complications associated with nonsteroidal anti-inflammatory drugs, glucocorticoids, acetaminophen, and combinations of these agents". Arthritis Research and Therapy 3: 98. doi:. PMID 11178116.
- ^ Rudolph AM (February 1981). "Effects of aspirin and acetaminophen in pregnancy and in the newborn". Arch. Intern. Med. 141 (3 Spec No): 358–63. PMID 7469626.
- ^ Lesko SM, Mitchell AA (October 1999). "The safety of acetaminophen and ibuprofen among children younger than two years old". Pediatrics 104 (4): e39. PMID 10506264. http://pediatrics.aappublications.org/cgi/pmidlookup?view=long&pmid=10506264.
- ^ Colás Chacartegui R, Temprano González R, Gómez Arruza C, Muñoz Cacho P, Pascual Gómez J (2005). "[Abuse pattern of analgesics in chronic daily headache: a study in the general population]". Rev Clin Esp 205 (12): 583–87. PMID 16527179.
- ^ Mohandas J, Duggin GG, Horvath JS, Tiller DJ (November 1981). "Metabolic oxidation of acetaminophen (paracetamol) mediated by cytochrome P-450 mixed-function oxidase and prostaglandin endoperoxide synthetase in rabbit kidney". Toxicol. Appl. Pharmacol. 61 (2): 252–9. PMID 6798713. http://linkinghub.elsevier.com/retrieve/pii/0041-008X(81)90415-4.
- ^ Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB (October 1973). "Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione". The Journal of pharmacology and experimental therapeutics 187 (1): 211–7. PMID 4746329. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=4746329.
- ^ Ryder SD, Beckingham IJ (2001). "ABC of diseases of liver, pancreas, and biliary system. Other causes of parenchymal liver disease". BMJ 322 (7281): 290–92. doi:. PMID 11157536. [11157536 Free full text]
- ^ Lee WM (July 2004). "Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure". Hepatology 40 (1): 6–9. doi:. PMID 15239078. http://www3.interscience.wiley.com/cgi-bin/fulltext/109086434/PDFSTART.
- ^ Allen AL (2003). "The diagnosis of acetaminophen toxicosis in a cat". Can Vet J 44 (6): 509–10. PMID 12839249.
- ^ Rumbeiha WK, Lin YS, Oehme FW (November 1995). "Comparison of N-acetylcysteine and methylene blue, alone or in combination, for treatment of acetaminophen toxicosis in cats". Am. J. Vet. Res. 56 (11): 1529–33. PMID 8585668.
- ^ a b Maddison, Jill E.; Stephen W. Page, David Church (2002). Small Animal Clinical Pharmacology. Elsevier Health Sciences. pp. 260–261. ISBN 0702025739.
- ^ "Pardale-V Tablets: Presentation". UK National Office of Animal Health Compendium of Animal Medicines. September 28, 2006. http://www.noahcompendium.co.uk/Dechra/Pardale-V_Oral_Tablets/-27619.html. Retrieved on 03 2007accessmonth=01.
- ^ "Pardale-V Tablets: Legal Category". UK National Office of Animal Health Compendium of Animal Medicines. November 15, 2005. http://www.noahcompendium.co.uk/Dechra/Pardale-V_Oral_Tablets/-27624.html. Retrieved on 03 01 2007.
- ^ Villar D, Buck WB, Gonzalez JM (1998). "Ibuprofen, aspirin and acetaminophen toxicosis and treatment in dogs and cats". Vet Hum Toxicol 40 (3): 156–62. PMID 9610496.
- ^ Johnston J, Savarie P, Primus T, Eisemann J, Hurley J, Kohler D (2002). "Risk assessment of an acetaminophen baiting program for chemical control of brown tree snakes on Guam: evaluation of baits, snake residues, and potential primary and secondary hazards". Environ Sci Technol 36 (17): 3827–33. doi:. PMID 12322757.
[edit] External links
- Paracetamol Information Centre
- The Julius Axelrod Papers
- FDA: Safe Use of Over-the-Counter Pain Relievers/Fever Reducers
- FDA: Consumer education on Pain Relievers/Fever Reducers
- Manning AM. "Acetaminophen Toxicity in Dogs". PetPlace.com. http://www.petplace.com/dogs/acetaminophen-toxicity-in-dogs/page1.aspx. Retrieved on 17 01 2009.


