Inositol
From Wikipedia, the free encyclopedia
| myo-Inositol[1] | |
|---|---|
 |
|
 |
|
| IUPAC name |
|
| Other names | (1r,2R,3S,4s,5R,6S)-cyclohexane-1,2,3,4,5,6-hexol, Cyclohexanehexol, Cyclohexitol, Dambose, Inosital, Inosite, iso-Inositol, Inositene, Inositina, i-Inositol, Inositol, MI, Meat sugar, Mesoinosit, Mesoinosite, meso-Inositol, Mesol, Mesovit, Myoinosite, Mouse antialopecia factor, Nucite, Phaseomannite, Phaseomannitol, Rat antispectacled eye factor, and Scyllite (for the structural isomer scyllo-Inositol) |
| Identifiers | |
| CAS number | 87-89-8 |
| SMILES |
|
| Properties | |
| Molecular formula | C6H12O6 |
| Molar mass | 180.16 g mol−1 |
| Density | 1.752 g/cm³ |
| Melting point |
225-227 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Inositol, (of which the most prominent naturally occurring form is myo-inositol, cis-1,2,3,5-trans-4,6-cyclohexanehexol), is a carbocyclic polyol that plays an important role as the structural basis for a number of secondary messengers in eukaryotic cells, including inositol phosphates, phosphatidylinositol (PI) and phosphatidylinositol phosphate (PIP) lipids. It is found in many foods, in particular, in cereals with high bran content, nuts, beans, and fruit, especially cantaloupe melons and oranges. Inositol is not considered a vitamin itself because it can be synthesised by the body.
Other naturally occurring isomers (though in minimal quantities) are scyllo-, chiro-, muco-, and neo-inositol. Other possible isomers are allo-, epi-, and cis-inositol.
Myo-Inositol was classified as a member of the vitamin B complex (often referred to as vitamin B8), but was found to be synthesized by the human body (thus, declassifying it as a vitamin). It should be noted, however, that substances such as niacin and choline can also be synthesized in the body, but are not made in amounts considered adequate for good health, and are classified as essential nutrients.
Contents |
[edit] Structure
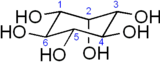
The chemical formula of myo-inositol is C6H12O6. In its most stable geometry, the inositol ring is in the chair conformation. There are nine stereoisomers, all of which may be referred to as inositol; however, the natural isomer has a structure in which the 1st, 3rd, 4th, 5th, and 6th hydroxyls are equatorial, whereas the 2nd hydroxyl group is axial.[2]
[edit] Synthesis
Myo-Inositol is synthesized from glucose-6-phosphate (G-6-P) in two steps. First, G-6-P is isomerised by ISYNA1 to myo-inositol 1-phosphate, which is then dephosphorylated by IMPase 1 to give free myo-inositol.
[edit] Function
Inositol as the basis for a number of signaling and secondary messenger molecules, is involved in a number of biological processes, including:
- Insulin signal transduction[3]
- Cytoskeleton assembly
- Nerve guidance (Epsin)
- Intracellular calcium (Ca2+) concentration control[citation needed]
- Cell membrane potential maintenance[citation needed]
- Serotonin activity modulation
- Breakdown of fats and reducing blood cholesterol[citation needed]
- Gene expression[4][5]
[edit] Clinical implications
[edit] Psychiatric conditions
Patients suffering from clinical depression generally have decreased levels of inositol in their cerebrospinal fluid.[6]
Some preliminary results of studies on inositol supplements show promising results for people suffering from problems such as bulimia, panic disorder, obsessive-compulsive disorder, and unipolar and bipolar depression.[6]
Myo-inositol has been found in a single double-blind study to significantly reduce the symptoms of obsessive-compulsive disorder (OCD) with effectiveness equal to SSRIs and virtually without side-effects.[7] In a double-blind, controlled trial, myo-inositol was superior to fluvoxamine for decreasing the number of panic attacks and had fewer side effects.[8] A double-blind, placebo-controlled study of depressed patients showed that a high dose of inositol (12 grams daily) resulted in significant improvement of symptoms, with no changes noted in liver, kidney, or hematological function.[6]
Lithium treatment has been found to inhibit the enzyme inositol monophosphatase leading to higher levels of inositol triphosphate.[9] This effect was enhanced further with an inositol triphosphate reuptake inhibitor.
[edit] Other conditions
D-chiro-inositol (DCI) has been found in two double-blind studies to be an effective treatment for many of the clinical hallmarks of polycystic ovary syndrome (PCOS), including insulin resistance, hyperandrogenism, and oligo-amenorrhea.[10][11] The impetuses for these studies were the observed defects in DCI metabolism in PCOS and the implication of DCI in insulin signal transduction.[12][13]
Animal studies suggest inositol reduces the severity of the osmotic demyelination syndrome if given prior to rapid correction of chronic hyponatraemia.[14] Further study is required prior to its application in humans for this indication.
Studies from in vitro experiments, animal studies, and limited clinical experiences, claim that inositol may be used effectively against some types of cancer, in particular, when used in combination with phytic acid.[15]
[edit] Use in Hair Care
According to some reports, Inositol strengthens the cells of the hair helping it to retain moisture. It is available orally and is an ingredient in some shampoo formulas. [16]
[edit] Common use as a "cutting" agent
Inositol is also commonly used as an adulterant (or cutting agent) in many illegal drugs, such as cocaine, methamphetamine, and sometimes heroin.[17].
[edit] See also
- D-chiro-inositol
- Essential nutrient
- Hyperuricemia
- Hypouricemia
- Inositol phosphate
- Inositol trisphosphate
- Inositol pentakisphosphate
- Inositol hexaphosphate
- Inositol triphosphate receptor
- Inositol hexanicotinate
[edit] References
- ^ Merck Index, 11th Edition, 4883.
- ^ The Chemical and Bio-physical properties of Phosphatidylinositol phosphates, Thesis for M.Res.. Imperial College London. 2006.
- ^ Larner J (2002). "D-chiro-inositol--its functional role in insulin action and its deficit in insulin resistance". Int J Exp Diabetes Res 3 (1): 47–60. doi:. PMID 11900279.
- ^ Shen, X. (2003). "Modulation of ATP-dependent chromatin-remodeling complexes by inositol polyphosphates". Science 299 (5603): 112–4. doi:. PMID 12434013.
- ^ Steger, D. J. (2003). "Regulation of chromatin remodelling by inositol polyphosphates". Science 299 (5603): 114–6. doi:. PMID 12434012.
- ^ a b c Nick, Gina L. (2004). "Inositol as a treatment for psychiatric disorders: a scientific evaluation of its clinical effectiveness". Townsend Letter for Doctors and Patients (October). http://findarticles.com/p/articles/mi_m0ISW/is_255/ai_n6211958. Retrieved on 2008-05-24.
- ^ Fux M, Levine J, Aviv A, Belmaker RH (1996). "Inositol treatment of obsessive-compulsive disorder". American Journal of Psychiatry 153 (9): 1219–21. PMID 8780431.
- ^ Palatnik A, Frolov K, Fux M, Benjamin J (2001). "Double-blind, controlled, crossover trial of inositol versus fluvoxamine for the treatment of panic disorder". Journal of Clinical Psychopharmacology 21 (3): 335–339. doi:. PMID 11386498.
- ^ Einat H, Kofman O, Itkin O, Lewitan RJ, Belmaker RH (1998). "Augmentation of lithium's behavioral effect by inositol uptake inhibitors". J Neural Transm 105 (1): 31–8. PMID 9588758. http://link.springer.de/link/service/journals/00702/bibs/8105001/81050031.htm.
- ^ Nestler J E, Jakubowicz D J, Reamer P, Gunn R D, Allan G (1999). "Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome". N Engl J Med 340 (17): 1314–1320. doi:. PMID 10219066.
- ^ Iuorno M J, Jakubowicz D J, Baillargeon J P, Dillon P, Gunn R D, Allan G, Nestler J E (2002). "Effects of d-chiro-inositol in lean women with the polycystic ovary syndrome". Endocr Pract 8 (6): 417–423. PMID 15251831.
- ^ Larner J (2002). "D-chiro-inositol--its functional role in insulin action and its deficit in insulin resistance". Int J Exp Diabetes Res 3 (1): 47–60. doi:. PMID 11900279.
- ^ Nestler J E, Jakubowicz D J, Iuorno M J (2000). "Role of inositolphosphoglycan mediators of insulin action in the polycystic ovary syndrome". J Pediatr Endocrinol Metab 13 Suppl 5: 1295–1298. PMID 11117673.
- ^ Silver SM, Schroeder BM, Sterns RH, Rojiani AM (2006). "Myoinositol administration improves survival and reduces myelinolysis after rapid correction of chronic hyponatremia in rats". J Neuropathol Exp Neurol 65 (1): 37–44. doi:. PMID 16410747.
- ^ jn.nutrition.org
- ^ http://www.natural-connection.com/resource/tnc_reference_library/aubrey_dictionary.html
- ^ http://www.examiner.com/x-281-Caffeine-Examiner~y2008m5d22-Ingredient-of-the-day-Guarana--Murdered-Child-Eyeballs

