Hippocampus
From Wikipedia, the free encyclopedia
| Brain: Hippocampus | ||
|---|---|---|
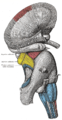
| The hippocampus is located in the medial temporal lobe of the brain. (In this lateral view of the human brain, the frontal lobe is at the left, the occipital lobe is at the right, and the temporal lobe has largely been removed to reveal the hippocampus underneath.) | ||
| NeuroNames | hier-164 | |
| MeSH | Hippocampus | |
The hippocampus is a major component of the brains of humans and other mammals. It belongs to the limbic system and plays major roles in long term memory and spatial navigation. Like the cerebral cortex, it is a paired structure, with mirror-image halves in the left and right sides of the brain. The hippocampus is closely associated with the cerebral cortex, and in primates is located in the medial temporal lobe, underneath the cortical surface. It is shaped like a curved tube, which in humans is convoluted in a way that reminded early anatomists of a seahorse. The name, in fact, derives from the Greek word for seahorse (Greek: ιππος, hippos = horse, καμπος, kampos = sea monster).
In Alzheimer's disease the hippocampus is one of the first regions of the brain to suffer damage; memory problems and disorientation appear among the first symptoms. Damage to the hippocampus can also result from oxygen starvation (anoxia), encephalitis, or medial temporal lobe epilepsy. People with extensive hippocampal damage may experience amnesia, that is, inability to form or retain new memories.
In rodents, the hippocampus has been studied extensively as part of the brain system responsible for spatial memory and navigation. Many neurons in the rat and mouse hippocampus respond as place cells: that is, they fire bursts of action potentials when the animal passes through a specific part of its environment. Hippocampal place cells interact extensively with head direction cells, whose activity acts as an inertial compass, and with grid cells in the neighboring entorhinal cortex.
Because of its densely packed layers of neurons, the hippocampus has frequently been used as a model system for studying neurophysiology. The form of neural plasticity known as long term potentiation (LTP) was originally discovered in the hippocampus, and has often been studied there. LTP is widely believed to be one of the main neural mechanisms by which memory is stored in the brain.
Contents |
[edit] Functions of the hippocampus
Historically, the earliest widely held hypothesis was that the hippocampus is involved in olfaction. This idea was largely motivated by a belief, later shown to be false, that the hippocampus receives direct input from the olfactory bulb.[1] There continues to be some interest in hippocampal olfactory responses, particular the role of the hippocampus in memory for odors, but few people believe today that olfaction is a primary function of the hippocampus[2].
Over the years, three main ideas of hippocampal function have dominated the literature: inhibition, memory, and space. The behavioral inhibition theory (caricatured by O'Keefe and Nadel as "slam on the brakes!"[3]) was very popular up to the 1960s. It derived much of its force from two observations: first, that animals with hippocampal damage tend to be hyperactive; second, that animals with hippocampal damage often have difficulty learning to inhibit responses that they have previously been taught. Jeffrey Gray developed this line of thought into a full-fledged theory of the role of the hippocampus in anxiety[4]. The inhibition theory is currently the least popular of the three.
The second important line of thought relates the hippocampus to memory. Although it had precursors, this idea derived its main force from a famous report by Scoville and Milner[5] of the results of surgical destruction of the hippocampus (in an attempt to relieve epileptic seizures), in a patient named Henry Gustav Molaison,[6], known until his death in 2008 as H.M.. The unexpected outcome of the surgery was severe anterograde and partial retrograde amnesia: H.M. was unable to form new episodic memories after his surgery and could not remember any events that occurred just before his surgery, but retained memories for things that happened years earlier, such as his childhood. This case produced such enormous interest that H.M. became the most intensively studied medical case in history. In the ensuing years, other patients with similar levels of hippocampal damage and amnesia (caused by accident or disease) have been studied as well, and thousands of experiments have studied the physiology of neural plasticity in the hippocampus. There is now almost universal agreement that the hippocampus plays some sort of important role in memory; however, the precise nature of this role remains widely debated[7][8].
The third important line of thought relates the hippocampus to space. The spatial theory was originally championed by O'Keefe and Nadel, who were influenced by E. C. Tolman's theories about "cognitive maps" in humans and animals. O'Keefe and his student Dostrovsky in 1971 discovered neurons in the rat hippocampus that appeared to them to show activity that encoded the rat's location within its environment. O'Keefe and his co-workers, especially Lynn Nadel, continued to investigate this question, in a line of work that eventually led to their very influential 1978 book called "The hippocampus as a cognitive map"[9]. As with the memory theory, there is now almost universal agreement that spatial coding somehow plays an important role in hippocampal function, but the details are widely debated.
[edit] Role in memory
Psychologists and neuroscientists generally agree that the hippocampus has an important role in the formation of new memories about experienced events (episodic or autobiographical memory)[8][10]. Some researchers view the hippocampus as part of a larger medial temporal lobe memory system responsible for general declarative memory (memories that can be explicitly verbalized — these would include, for example, memory for facts in addition to episodic memory)[7].
Severe damage to the hippocampus results in profound difficulties in forming new memories (anterograde amnesia), and often also affects memories formed prior to the damage (retrograde amnesia). Although the retrograde effect normally extends some years prior to the brain damage, in some cases older memories remain - this sparing of older memories leads to the idea that consolidation over time involves the transfer of memories out of the hippocampus to other parts of the brain.[11]
Damage to the hippocampus does not affect some types of memory, such as the ability to learn new motor or cognitive skills (playing a musical instrument, or solving certain types of puzzles, for example). This fact suggests that such abilities depend on different types of memory (procedural memory) and different brain regions. Furthermore, amnesic patients frequently show "implicit" memory for experiences even in the absence of conscious knowledge. For example, a patient asked to guess which of two faces he has seen most recently may give the correct answer the majority of the time, in spite of stating that he has never seen either of the faces before. Some researchers distinguish between conscious recollection, which depends on the hippocampus, and familiarity, which depends on portions of the medial temporal cortex.[12]
[edit] Role in spatial memory and navigation

Studies conducted on rats and mice have shown that many neurons in the hippocampus have spatial firing fields, that is, they fire bursts of action potentials when a rat passes through a particular part of the environment. In some cases, the firing rate varies depending on the direction a rat is moving, the destination toward which it is traveling, or other task-related variables.[14] When rats are travel along stereotyped trajectories, repeating the same path over and over, hippocampal activity is strongly direction-dependent, but when rats freely explore open areas, the activity is largely non-directional. Evidence for place cells in primates is limited, perhaps in part because it is difficult to record brain activity from freely moving monkeys. In humans, place cells have been reported in a study of epileptic patients with diagnostic electrodes implanted in the hippocampus for purposes of localizing the sources of seizures—the patients were using a computer to move around in a virtual reality town.[15]
The discovery of place cells in the 1970s led to a theory that the hippocampus might act as a cognitive map — a neural representation of the layout of the environment.[16] Studies with animals have shown that an intact hippocampus is required for some spatial memory tasks, particularly ones that require finding the way to a hidden goal)[17].
Without a fully functional hippocampus, humans may not remember where they have been and how to get where they are going: getting lost is one of the most common symptoms of amnesia. Brain imaging shows that people have more active hippocampi when correctly navigating, as tested in a computer-simulated "virtual" navigation task[18]. Also, there is evidence that the hippocampus plays a role in finding shortcuts and new routes between familiar places. For example, London's taxi drivers must learn a large number of places and the most direct routes between them (they have to pass a strict test, The Knowledge, before being licensed to drive the famous black cabs). A study at University College London by Maguire, et al (2000)[19] showed that part of the hippocampus is larger in taxi drivers than in the general public, and that more experienced drivers have bigger hippocampi. Whether having a bigger hippocampus helps an individual to become a cab driver or finding shortcuts for a living makes an individual's hippocampus grow is yet to be elucidated. However, in that study Maguire, et al examined the correlation between size of the grey matter and length of time that had been spent as a taxi driver, and found that the longer an individual had spent as a taxi driver, the larger the volume of the right hippocampus. It was found that the total volume of the hippocampus remained constant, from the control group vs. taxi drivers. That is to say that the posterior portion of a taxi driver's hippocampus is indeed increased, but at the expense of the anterior portion. There have been no known detrimental effects reported from this disparity in hippocampal proportions. [19] This finding suggested to the authors that the hippocampus increases in size with use over time.[19]
[edit] Anatomy
Anatomically, the hippocampus is an elaboration of the edge of the cortex. It can be distinguished as a zone where the cortex narrows into a single layer of very densely packed neurons, which curls into a tight S shape. The structures that line the edge of the cortex make up the so-called limbic system (Latin limbus = border): these include the hippocampus, cingulate cortex, olfactory cortex, and amygdala. Paul MacLean once suggested, as part of his triune brain theory, that the limbic structures comprise the neural basis of emotion. Most neuroscientists no longer believe that the concept of a unified "limbic system" is valid, though.
The hippocampus, as a whole, has the shape of a curved tube, which has been analogized variously to a seahorse, or a ram's horn (Cornu Ammonis), or a banana. It consists of a ventral and dorsal portion, both of which share similar composition but are parts of completely different neural circuits. This general layout holds across the full range of mammalian species, from hedgehog to human, although the details vary. In the rat, the two hippocampi look astonishingly like a pair of bananas, joined at the stem. In human or monkey brains, the portion of the hippocampus down at the bottom, near the base of the temporal lobe, is much broader than the part at the top. One of the consequences of this complex geometry is that cross-sections through the hippocampus can show a variety of shapes, depending on the angle and location of the cut.
The strongest connections of the hippocampus are with the entorhinal cortex (EC), which lies next to it in the temporal lobe. The superficial layers of the EC provide the most numerous inputs to the hippocampus, and the deep layers of the EC receive the most numerous outputs. The EC, in turn, is strongly, and reciprocally, connected with many other parts of the cortex. The hippocampus also receives a very important projection from the medial septal area. Destruction of the septal area abolishes the hippocampal theta rhythm, and severely impairs certain types of memory. (So-called "date rape" drugs are thought to exert their amnestic effects at least partly by antagonizing the cholinergic projection from the medial septum to the hippocampus.)
[edit] Physiology

The hippocampus shows two major "modes" of activity, each associated with a distinct pattern of EEG waves and neural population activity. These modes are named after the EEG patterns associated with them: theta and large irregular activity (LIA). Here are some of their main characteristics in the rat, the animal that has been most extensively studied:[20]
The theta mode appears during states of active, alert behavior (especially locomotion), and also during REM (dreaming) sleep. In the theta mode, the EEG is dominated by large regular waves with a frequency range of 6–9 Hz, and the main groups of hippocampal neurons (pyramidal cells and granule cells) show sparse population activity, which means that in any short time interval, the great majority of cells are silent, while the small remaining fraction fire at relatively high rates, up to 50 spikes in one second for the most active of them. An active cell typically stays active for from half a second to a few seconds. As the rat behaves, the active cells fall silent and new cells become active, but the overall percentage of active cells remains more or less constant. In many situations, cell activity is determined largely by the spatial location of the animal, but other behavioral variables also clearly influence it.
The LIA mode appears during slow-wave (non-dreaming) sleep, and also during states of waking immobility, such as resting or eating. In the LIA mode, the EEG is dominated by sharp waves, which are randomly-timed large deflections of the EEG signal lasting for 200-300 msec. These sharp waves also determine the population neural activity patterns. Between them, pyramidal cells and granule cells are very quiet (but not silent). During a sharp wave, as many as 5-10% of the population may emit action potentials during a period of 50 msec; many of these cells emit not one but a burst of spikes.
These two hippocapampal activity modes can be seen in primates as well as rats, with the important exception that it has been difficult to see robust theta rhythmicity in the primate hippocampus. There are, however, qualitatively similar sharp waves, and similar state-dependent changes in neural population activity.[21].
[edit] The theta rhythm
Because of its densely packed neural layers, the hippocampus generates some of the largest EEG signals of any brain structure. In some situations the EEG is dominated by regular waves at 3-10 Hz, often continuing for many seconds. This EEG pattern is known as the theta rhythm. It was one of the earliest EEG phenomena to be discovered: the first description came from Jung and Kornmuller, in 1938. It was not until 1954, however, with the publication by Green and Arduini of a long and thorough study of theta rhythm in rabbits, cats, and monkeys, that interest really took off.[22] Perhaps largely because they related the theta rhythm to arousal, which was the hot topic of the day, their paper provoked a flood of followup studies, resulting in the publication of literally hundreds of studies of the physiology and pharmacology of theta during the 1950s and 1960s. In spite of this rather daunting body of work, many questions remained unanswered, especially the question of function. Even at present this most critical of questions has not yet been convincingly answered.
Theta rhythmicity is very obvious in rabbits and rodents, and also clearly present in cats and dogs. Whether theta can be seen in primates is a vexing question. Green and Arduini reported only very short bursts of rather irregular rhythmicity in monkeys, and most later studies have seen little more. However, variations in methodology have made it difficult to draw strong conclusions.[23]
In rats (the animals that have been by far the most extensively studied), theta is seen mainly in two conditions: first, when an animal is walking or in some other way actively interacting with its surroundings; second, during REM sleep.[24] The frequency increases as a function of running speed, starting at about 6.5 Hz on the low end, and increasing to about 9 Hz on the high end, although higher frequencies are sometimes seen for dramatic movements such as jumps across wide gaps. In other, larger, species of animals, theta frequencies are generally a bit lower. The behavioral dependency also seems to vary by species: in cats and rabbits, theta is often observed during states of motionless alertness. This has been reported for rats as well, but only when they are severely frightened.[25]
Theta is not just confined to the hippocampus. In rats, it can be observed in many parts of the brain, including nearly all that interact strongly with the hippocampus. The pacemaker for the rhythm is thought to lie within the medial septal area: this area projects to all of the regions that show theta rhythmicity, and destruction of it eliminates theta throughout the brain. (There may be one exception, a small area in the hypothalamus called the supramamillary nucleus, which seems to be capable of sustaining theta independently of the septum in some situations.[26])
The function of theta, presuming it has one, has not yet been convincingly explained, although numerous theories have been proposed.[27] The most popular trend has been to relate it to learning and memory. It is well established that lesions of the medial septum---the central node of the theta system---cause severe disruptions of memory. However, the medium septum is more than just the controller of theta, it is also the main source of cholinergic projections to the hippocampus. It has not been established that septal lesions exert their effects specifically by eliminating theta.
[edit] Sharp waves
During sleep, or during waking states when an animal is resting or otherwise not engaged with its surroundings, the hippocampal EEG shows a pattern of irregular slow waves, somewhat larger in amplitude than theta waves. This pattern is occasionally interrupted by large surges called sharp waves. These events are associated with bursts of spike activity, lasting 50-100 msec, in pyramidal cells of CA3 and CA1. They are also associated with short-lasting high-frequency EEG oscillations called "ripples". Ripples, with frequencies in the range 150-200 Hz in rats, can usually be detected only by electrodes located either inside, or very close to, the CA1 cell body layer. In contrast, electrodes located anywhere inside the hippocampus, or even in neighboring brain structures, will often pick up sharp waves as large slow EEG deflections, lasting 200-400 msec.
In rats, sharp waves are most robust during sleep, when they occur at an average rate around 1 per second, but in a very irregular temporal pattern. Sharp waves also occur during inactive waking states, but they are less frequent then and usually smaller. Sharp waves have also been observed in the human temporal lobe and monkey hippocampus. In monkeys, sharp waves are quite robust, but do not occur nearly as frequently as in rats.
One of the most interesting aspects of sharp waves is that they appear to be associated with memory. Wilson and McNaughton 1994, and numerous later studies, reported that when hippocampal place cells have overlapping spatial firing fields (and therefore often fire in near-simultaneity), they tend to show correlated activity during sleep following the behavioral session. This enhancement of correlation, commonly known as reactivation, has been found to be confined mainly to sharp waves. It has been proposed that sharp waves are, in fact, reactivations of neural activity patterns that were memorized during behavior, driven by strengthening of synaptic connections within the hippocampus. This idea forms a key component of the "two-stage memory" theory, advocated by Buzsaki and others, which proposes that memories are stored within the hippocampus during behavior, and then later transferred to the neocortex during sleep: sharp waves are suggested to drive Hebbian synaptic changes in the neocortical targets of hippocampal output pathways.
[edit] Role in epilepsy
The hippocampus is often the focus of epileptic seizures: hippocampal sclerosis is the most commonly visible type of tissue damage in temporal lobe epilepsy.[28] It is not yet clear, though, whether the epilepsy is usually caused by hippocampal abnormalities, or the hippocampus is damaged by cumulative effects of seizures. In experimental settings where repetitive seizures are artificially induced in animals, hippocampal damage is a frequent result: this may be a consequence of the hippocampus being one of the most electrically excitable parts of the brain. It may also have something to do with the fact that the hippocampus is one of very few brain regions where new neurons continue to be created throughout life.
[edit] Evolution
The hippocampus has a generally similar appearance across the range of mammal species, from basal ones such as the hedgehog to the most "advanced" ones such as humans[29]. The hippocampal-size-to-body-size ratio broadly increases, being about twice as large for primates as for the hedgehog. It does not, however, increase at anywhere close to the rate of the neocortex-to-body-size ratio. Thus, the hippocampus takes up a much larger volume of the cortical mantle in rodents than in primates.
There is also a general relationship between the size of the hippocampus and spatial memory: when comparisons are made between similar species, ones that have a greater capacity for spatial memory tend to have larger hippocampal volumes.[30]. This relationship also extends to sex differences: in species where males and females show strong differences in spatial memory ability, they also tend to show corresponding differences in hippocampal volume[31]
Non-mammalian species do not have a brain structure that looks like the mammalian hippocampus, but they have one that is considered homologous to it. The hippocampus, as pointed out above, is essentially the medial edge of the cortex. Only mammals have a fully developed cortex, but the structure it evolved from, called the pallium, is present in all vertebrates, even the most primitive ones such as the lamprey or hagfish[32]. The pallium is usually divided into three zones: medial, lateral, and dorsal. The medial pallium forms the precursor of the hippocampus. It does not resemble the hippocampus visually, because the layers are not warped into an S shape or enwrapped by the dentate gyrus, but the homology is indicated by strong chemical and functional affinities. There is now evidence that these hippocampal-like structures are involved in spatial cognition in birds, reptiles, and fish.[33]
In birds, the correspondence is sufficiently well established that most anatomists refer to the medial pallial zone as the "avian hippocampus".[34] Numerous species of birds have strong spatial skills, particularly those that cache food. There is evidence that food-caching birds have a larger hippocampus than other types of birds, and that damage to the hippocampus causes impairments in spatial memory.[35].
The story for fish is more complex. In teleost fish (which make up the great majority of existing species), the forebrain is weirdly distorted in comparison to other types of vertebrates. Most neuroanatomists believe that the teleost forebrain is essentially everted, like a sock turned inside-out, so that structures that lie in the interior, next to the ventricles, for most vertebrates, are found on the outside in teleost fish, and vice versa.[36] One of the consequences of this is that the medial pallium ("hippocampal" zone) of a typical vertebrate is thought to correspond to the lateral pallium of a typical fish. Several types of fish (particularly goldfish) have been shown experimentally to have strong spatial memory abilities, even forming "cognitive maps" of the areas they inhabit.[30] There is evidence that damage to the lateral pallium impairs spatial memory.[37] [38] (Long-distance navigation, such as homing by salmon, seems to rely on different mechanisms, however.)
Thus, the role of the hippocampal region in navigation appears to begin far back in vertebrate evolution, predating splits that occurred hundreds of millions of years ago.[39] It is not yet known whether the medial pallium plays a similar role in even more primitive vertebrates, such as sharks and rays, or even lampreys and hagfish. Some types of insects, and molluscs such as the octopus, also have strong spatial learning and navigation abilities, but these appear to work differently from the mammalian spatial system, so there is as yet no good reason to think that they have a common evolutionary origin; nor is there sufficient similarity in brain structure to enable anything resembling a "hippocampus" to be identified in these species. Some have proposed, though, that the insect's mushroom bodies may have a function similar to that of the hippocampus.[40]
[edit] Additional images
|
Superficial dissection of brain-stem. Lateral view. |
The fornix and corpus callosum from below. |
|||
|
Hippocampal areas in a Nissl-stained coronal section of the rat brain. DG: Dentate gyrus. |
[edit] See also
[edit] Notes
- ^ Finger, p. 183
- ^ Eichenbaum et al, 1991
- ^ Nadel et al, 1975
- ^ Gray and McNaughton, 2000
- ^ Scoville and Milner, 1957
- ^ "H. M., an Unforgettable Amnesiac, Dies at 82 - Obituary (Obit) - NYTimes.com". http://www.nytimes.com/2008/12/05/us/05hm.html?_r=1. Retrieved on 2008-12-06.
- ^ a b Squire, 1992
- ^ a b Eichenbaum and Cohen, 1993
- ^ O'Keefe and Nadel, 1978
- ^ Squire and Schacter, 2002
- ^ Squire and Schacter, 2002, Ch. 1
- ^ Diana et al, 2007
- ^ Skaggs et al., 1996
- ^ Smith and Mizumori, 2006
- ^ Ekstrom et al., 2003
- ^ O'Keefe and Nadel
- ^ Morris et al., 1982
- ^ Maguire et al., 1998
- ^ a b c Maguire et al., 2000
- ^ Buzsaki, 2006
- ^ Skaggs et al., 2007
- ^ Green and Arduini, 1954
- ^ Cantero et al., 2003
- ^ Vanderwolf, 1969
- ^ Sainsbury et al., 1987
- ^ Kirk and McNaughton, 1991
- ^ Buzsáki, 2006
- ^ Chang and Lowenstein, 2003
- ^ West, 1990
- ^ a b Jacobs, 2003
- ^ Jacobs et al., 1990
- ^ Aboitiz et al., 2003
- ^ Rodríguez et al., 2002
- ^ Colombo and Broadbent, 2000
- ^ Shettleworth, 2003
- ^ Nieuwenhuys, 1982
- ^ Portavella et al., 2002
- ^ Vargas et al., 2006
- ^ Broglio et al., 2005
- ^ Mizunami M, Weibrecht JM, Strausfeld NJ (December 1998). "Mushroom bodies of the cockroach: their participation in place memory". J. Comp. Neurol. 402 (4): 520–37. doi:. PMID 9862324.
[edit] References
- Aboitiz, F; Morales D, Montiel J (2003). "The evolutionary origin of the mammalian isocortex: Towards an integrated developmental and functional approach". Behav. Brain Sciences 26: 535–552. doi:. PMID ref15179935.
- Amaral, D; Lavenex P (2006). "Ch 3. Hippocampal Neuroanatomy". in Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J. The Hippocampus Book. Oxford University Press. ISBN 9780195100273.
- Broglio, C; Gómez A, Durán E, Ocaña FM, Jiménez-Moya F, Rodríguez F, Salas C (2002). "Hallmarks of a common forebrain vertebrate plan: Specialized pallial areas for spatial, temporal and emotional memory in actinopterygian fish". Brain Res. Bull. 57: 397–399. PMID 16144602.
- Buzsáki, G (2002). "Theta oscillations in the hippocampus" (PDF). Neuron 33: 325–340. doi:. PMID 11832222. http://osiris.rutgers.edu/BuzsakiHP/Publications/PDFs/BuzsakiTheta.pdf.
- Buzsáki, G (2006) ([dead link] – Scholar search). Rhythms of the Brain. Oxford University Press. ISBN 0195301064. http://books.google.com/books?id=refyVz4d4d9ZzsC.
- Cantero, JL; Atienza M, Stickgold R, Kahana MJ, Madsen JR, Kocsis B (26 Nov 2003). "Sleep-dependent theta oscillations in the human hippocampus and neocortex". J. Neurosci. 23 (34): 10897–10903. PMID 14645485. http://www.jneurosci.org/cgi/content/full/23/34/10897.
- Chang, BS; Lowenstein DH (2003). "Epilepsy". N. Engl. J. Med. 349: 1257–1266. doi:. PMID 14507951. http://www.medical-journals.com/r03102b.htm.
- Colombo, M; Broadbent N (2000). "Is the avian hippocampus a functional homologue of the mammalian hippocampus?". Neurosci. Biobehav. Rev. 24: 465–484. doi:. PMID ref10817844.
- Diana RA, Yonelinas AP, Ranganath C (2007). "Imaging recollection and familiarity in the medial temporal lobe: a three-component model". Trends Cogn Sci 11: 379–86. doi:. PMID 17707683.
- Eichenbaum, H; Otto TA, Wible CG, Piper JM (1991). "Ch 7. Building a model of the hippocampus in olfaction and memory". in Davis JL, Eichenbaum H,. Olfaction. MIT Press. ISBN 9780262041249.
- Eichnbaum, H; Cohen NJ (1993). Memory, Amnesia, and the Hippocampal System. MIT Press.
- Ekstrom, AD; Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, Fried I (2003). "Cellular networks underlying human spatial navigation" (PDF). Nature 425: 184–188. doi:. PMID 12968182. http://memory.psych.upenn.edu/publications/files/EkstEtal03.pdf.
- Finger, S (2001). Origins of Neuroscience: A History of Explorations Into Brain Function. Oxford University Press US. ISBN 9780195146943.
- Gray, JA; McNaughton N (2000). The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. Oxford University Press.
- Green, JD; Arduini AA (01 Nov 1954). "Hippocampal electrical activity in arousal". J. Neurophysiol. 17 (6): 533–557. PMID 13212425. http://jn.physiology.org/cgi/content/citation/17/6/533.
- Jacobs, LF; Gaulin SJ, Sherry DF, Hoffman GE (1990). "Evolution of spatial cognition: sex-specific patterns of spatial behavior predict hippocampal size". PNAS 87: 6349–6352. doi:. PMID ref2201026. http://www.pnas.org/cgi/reprint/87/16/6349.
- Jacobs, LF (2003). "The Evolution of the Cognitive Map". Brain Behav. Evol. 62: 128–139. doi:. PMID ref12937351.
- Kirk, IJ; McNaughton N (1991). "Supramammillary cell firing and hippocampal rhythmical slow activity". Neuroreport 11: 723–725. doi:. PMID 1810464.
- Maguire, EA; Burgess N, Donnett JG, Frackowiak RSJ, Firth CD, O'Keefe J (1998). "Knowing Where and Getting There: A Human Navigation Network". Science 280: 921–924. doi:. PMID 9572740. http://www.sciencemag.org/cgi/content/abstract/280/5365/921.
- Maguire, EA; Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD (2000). "Navigation-related structural change in the hippocampi of taxi drivers". PNAS 97: 4398–4403. doi:. PMID 10716738. http://www.pnas.org/cgi/content/full/97/8/4398.
- McNaughton, BL; Battaglia FP, Jensen O, Moser EI, Moser MB (2006). "Path integration and the neural basis of the 'cognitive map'". Nat. Rev. Neurosci. 7: 663–678. doi:. PMID 16858394. http://www.nature.com/nrn/journal/v7/n8/abs/nrn1932.html.
- Morris, RGM; Garrud P, Rawlins JNP, O'Keefe J (1982). "Place navigation impaired in rats with hippocampal lesions.". Nature 297: 681–683. doi:. PMID 7088155.
- Moser, EI; Kropf E, Moser M-B (2008). "Place Cells, Grid Cells, and the Brain's Spatial Representation System". Ann. Rev. Neurosci. 31: 69. doi:.
- Nadel L, O'Keefe J, Black A (1975). "Slam on the brakes: a critique of Altman, Brunner, and Bayer's response-inhibition model of hippocampal function". Behav Biol 14: 151–62. PMID 1137539.
- Nieuwenhuys, R (1982). "An Overview of the Organization of the Brain of Actinopterygian Fishes". Am. Zool. 22: 287–310. doi:.
- O'Kane, G; Kensinger EA, Corkin S (2004). "Evidence for semantic learning in profound amnesia: An investigation with patient H.M.". Hippocampus 14: 417–425. doi:. PMID 15224979. http://www3.interscience.wiley.com/cgi-bin/abstract/108562086/ABSTRACT.
- O'Keefe, J; Nadel L (1978). The Hippocampus as a Cognitive Map. Oxford University Press. http://www.cognitivemap.net/HCMpdf/HCMChapters.html.
- Portavella, M; Vargas JP, Torres B, Salas C (2002). "The effects of telencephalic pallial lesions on spatial, temporal, and emotional learning in goldfish". Brain Res. Bull. 57: 397–399. doi:. PMID 11922997.
- Rodríguez, F; Lópeza JC, Vargasa JP, Broglioa C, Gómeza Y, Salas C (2002). "Spatial memory and hippocampal pallium through vertebrate evolution: insights from reptiles and teleost fish". Brain Res. Bull. 57: 499–503. doi:. PMID ref11923018.
- Sainsbury, RS; Heynen A, Montoya CP (1987). "Behavioral correlates of hippocampal type 2 theta in the rat". Physiol. Behav. 39: 513–519. doi:. PMID 3575499.
- Scoville, WB; Milner B (1957). "Loss of Recent Memory After Bilateral Hippocampal Lesions". J. Neurol. Neurosurg. Psych. 20: 11–21. doi:. PMID 13406589. http://neuro.psychiatryonline.org/cgi/content/full/12/1/103.
- Shettleworth, SJ (2003). "Memory and Hippocampal Specialization in Food-Storing Birds: Challenges for Research on Comparative Cognition". Brain Behav. Evol. 62: 108–116. doi:. PMID ref12937349.
- Skaggs, WE; McNaughton BL, Wilson MA, Barnes CA (1996). "Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences". Hippocampus 6: 149–176. doi:. PMID ref8797016. http://www3.interscience.wiley.com/cgi-bin/abstract/72392/ABSTRACT.
- Smith DM, Mizumori SJ (2006). "Hippocampal place cells, context, and episodic memory". Hippocampus 16: 716–29. doi:. PMID 16897724.
- Squire, LR (1992). "Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans". Psych. Rev. 99: 195–231. doi:.
- Squire, LR; Schacter DL (2002). The Neuropsychology of Memory. Guilford Press.
- Vanderwolf, CH (1969). "Hippocampal electrical activity and voluntary movement in the rat". EEG & Clin. Neurophysiol. 26: 407–418. doi:. PMID 4183562. http://psycnet.apa.org/?fa=main.doiLanding&uid=ref1970-20296-001.
- Vargas, JP; Bingman VP, Portavella M, López JC (2006). "Telencephalon and geometric space in goldfish". Eur. J. Neurosci. 24: 2870–2878. doi:. PMID 17156211.
- West, MJ (1990). "Stereological studies of the hippocampus: a comparison of the hippocampal subdivisions of diverse species including hedgehogs, laboratory rodents, wild mice and men.". Prog. Brain Res. 83: 13–36. doi:. PMID 2203095.
[edit] External links
- Diagram of network
- Diagram of a Hippocampal Brain Slice
- Temporal-lobe.com An interactive diagram of the rat parahippocampal-hippocampal region
|
|||||||||||||