Anandamide
From Wikipedia, the free encyclopedia
| This article's factual accuracy may be compromised due to out-of-date information. Please help improve the article by updating it. There may be information on the talk page. |
| Anandamide | |
|---|---|
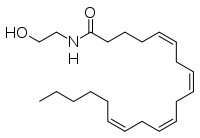 |
|
| IUPAC name |
|
| Other names | N-arachidonoylethanolamine arachidonoylethanolamide |
| Identifiers | |
| CAS number | 94421-68-8 |
| PubChem | |
| MeSH | |
| SMILES |
|
| Properties | |
| Molecular formula | C22H37NO2 |
| Molar mass | 347.53 g/mol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Anandamide, also known as N-arachidonoylethanolamine or AEA, is an endogenous cannabinoid neurotransmitter found in animal and human organs, especially in the brain. It was isolated and its structure was first described by Czech analytical chemist Lumír Ondřej Hanuš and American molecular pharmacologist William Anthony Devane in the Laboratory of Raphael Mechoulam, at the Hebrew University in Jerusalem, Israel in 1992. The name is taken from the Sanskrit word ananda, which means "bliss, delight", and amide.[1][2] It is degraded by the fatty acid amide hydrolase (FAAH) enzyme which converts anandamide into ethanolamine and arachidonic acid. As such, inhibitors of FAAH lead to elevated anandamide levels and are being pursued for therapeutic use.
Contents |
[edit] Cannabinoid receptors
Anandamide's effects can be either central, in the brain, or peripheral, in other parts of the body. These distinct effects are mediated primarily by CB1 cannabinoid receptors in the nervous system, and CB2 cannabinoid receptors in the periphery. The latter are mainly involved in functions of the immune system.
Cannabinoid receptors are part of the largest known family of receptors, the G protein-coupled receptors which span the cell membrane seven times. The CB1 receptor is one of the most abundant G protein-coupled receptors in the nervous system.
Cannabinoid receptors were originally discovered as being sensitive to Δ9-tetrahydrocannabinol (Δ9-THC, commonly called THC), which is the primary psychoactive cannabinoid found in cannabis. The discovery of anandamide came from research into CB1 and CB2, as it was inevitable that a naturally occurring (endogenous) chemical would be found to affect these receptors.
Anandamide has been shown to be involved in working memory.[3] Studies are under way to explore what role anandamide plays in human behavior, such as eating and sleep patterns, and pain relief.
Anandamide is also important for implantation of the early stage embryo in its blastocyst form into the uterus. Therefore cannabinoids such as Δ9-THC might interfere with the earliest stages of human pregnancy.[4]
Anandamide also is important in the regulation of feeding behavior, and the neural generation of motivation and pleasure. Both anandamide and exogenous cannabinoids like THC enhance food intake in animals and humans, an effect that is sometimes called the 'marijuana munchies.' In addition, anandamide injected directly into the forebrain reward-related brain structure nucleus accumbens enhances the pleasurable responses of rats to a rewarding sucrose taste, and enhances food intake as well[5].
Moreover, anandamide is thought to be an endogenous ligand for vanilloid receptors (which are involved in the transduction of acute and inflammatory pain signals), activating the receptor in a PKC-dependent (protein kinase C-dependent) manner.[citation needed]
[edit] Endogenous and dietary sources
Anandamide occurs in minute quantities in sea urchin roe.[6] Anandamide was reported to be present in chocolate[7] in small quantitities that were not assumed to have pharmacological or psychoactive effects.[8] However, a later study failed to repeat these findings and did not detect anandamide in chocolate.[9]
The human body synthesizes anandamide from N-arachidonoyl phosphatidylethanolamine, which is itself made by transferring arachidonic acid from phosphatidylcholine (PC) to the free amine of phosphatidylethanolamine (PE).[10][11] Endogenous anandamide is present at very low levels and has a very short half-life due to the action of the enzyme fatty acid amide hydrolase which breaks it down into free arachidonic acid and ethanolamine. Studies of piglets show that dietary levels of arachidonic acid and other essential fatty acids affect the levels of anandamide and other endocannabinoids in the brain.[12]
High fat diet feeding in mice increase levels of Anandamide in the liver and increase lipogenesis.[13] This indicates that Anandamide plays a role in the development of obesity, at least in rodents.
A study published in 1998 shows that anandamide inhibits human breast cancer cell proliferation.[14]
Paracetamol, or acetaminophen (in the U.S.A.) functions as a FAAH inhibitor. Subsequently, anandamide levels in the body and brain are elevated. This action may be partially or fully responsible for the analgesic effects of acetaminophen[citation needed].
[edit] See also
[edit] References
- ^ Devane W. A., Hanuš L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R.: Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946-1949 (1992)
- ^ Mechoulam R., Fride E.: The unpaved road to the endogenous brain cannabinoid ligands, the anandamides in “Cannabinoid Receptors” (ed. R. Pertwee), Academic Press, London. Pp. 233-258 (1995)
- ^ Mallet PE, Beninger RJ. The endogenous cannabinoid receptor agonist anandamide impairs memory in rats. Behav Pharmacol. 1996; 7:276-284.
- ^ Piomelli D. THC: moderation during implantation. Nat Med. 2004 Jan;10(1):19-20. PMID 14702623
- ^ Mahler SV, Smith KS, Berridge KC Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances 'liking' of a sweet reward. Neuropsychopharmacology. 2007 Nov;32(11):2267-78.
- ^ Bisogno T, Ventriglia M, Milone A, Mosca M, Cimino G, Di Marzo V. Occurrence and metabolism of anandamide and related acyl-ethanolamides in ovaries of the sea urchin Paracentrotus lividus. Biochim Biophys Acta. 1997 Apr 21;1345(3):338-48. PMID 9150253
- ^ E. di Tomaso, M. Beltramo, D. Piomelli (1996). "Brain cannabinoids in chocolate". Nature 382 (6593): 677–678. doi:. PMID 8751435.
- ^ Di Marzo V, Sepe N, De Petrocellis L, et al (1998). "Trick or treat from food endocannabinoids?". Nature 396 (6712): 636–7. doi:. PMID 9872309.
- ^ GC Willi, A Berger, V Di Marzo, T Bisogno, L De (2001). "Lipids in Neural Function: Modulation of Behavior by Oral Administration of Endocannabinoids Found in Foods". Nestle Nutr Workshop Ser Clin Perform Programme 5: 169–84; discussion 185–7. doi:. PMID 11510437.
- ^ V Natarajan, PV Reddy, PC Schmid, HH Schmid, N-Acylation of ethanolamine phospholipids in canine myocardium, Biochem. Biophys. Acta, 1982, Vol 712, 342-355, PMID 7126608
- ^ H Cadas, E di Tamaso, D Piomelli, Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain., J Neurosci, 1997, Vol 17(4), 1226-42. PMID 9006968
- ^ Alvin Berger, Anandamide and diet: Inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets, PNAS, May 22, 2001 vol. 9, no. 11, http://www.pnas.org/cgi/content/abstract/98/11/6402
- ^ Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Bátkai S, Harvey-White J, Mackie K, Offertáler L, Wang L, Kunos G, Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity, J Clin Invest. 2005 May;115(5):1298-305. PMID: 15864349
- ^ Luciano De Petrocellis, Dominique Melck, Antonella Palmisano, Tiziana Bisogno, Chiara Laezza, Maurizio Bifulco, Vincenzo Di Marzo (1998). "The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation". Proceedings of the National Academy of Sciences 95: 8375. doi:. PMID 9653194. http://www.pnas.org/cgi/content/full/95/14/8375?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&titleabstract=anandamide&searchid=1033146343712_4576&stored_search=&FIRSTINDEX=0&fdate=7/1/1998&tdate=7/31/1998.
[edit] External links
- Could anandamide be the missing link to "runner's high"? Accessed 2008-10-18


