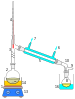
Distillation
From Wikipedia, the free encyclopedia
Distillation is a method of separating mixtures based on differences in their volatilities in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction.
Commercially, distillation has a number of uses. It is used to separate crude oil into more fractions for specific uses such as transport, power generation and heating. Water is distilled to remove impurities, such as salt from seawater. Air is distilled to separate its components—notably oxygen, nitrogen, and argon—for industrial use. Distillation of fermented solutions has been used since ancient times to produce distilled beverages with a higher alcohol content. The premises where distillation is carried out, especially distillation of alcohol, are known as a distillery.
Contents |
[edit] History
Early types of distillation were known to the Babylonians in Mesopotamia (in what is now Iraq) from at least the 2nd millennium BC.[1] Archaeological excavations in northwest Pakistan have yielded evidence that the distillation of alcohol was known in Pakistan since 500 BC,[2] but only became common between 150 BC - 350 AD.[2] Distillation was later known to Greek alchemists from the 1st century AD,[3][4][5] and the later development of large-scale distillation apparatus occurred in response to demands for spirits.[3] According to K. B. Hoffmann the earliest mention of "destillatio per descensum" occurs in the writings of Aetius, a Greek physician from the 5th century.[6] Hypatia of Alexandria is credited with having invented an early distillation apparatus,[7] and the first clear description of early apparatus for distillation is given by Zosimos of Panopolis in the fourth century.[5] Primitive tribes of India used a method of distillation for producing Mahuda liquor. This crude and ancient method is not very effective.[8]
The invention of highly effective "pure distillation" is credited to Arabic and Persian chemists in the Middle East from the 8th century. They produced distillation processes to isolate and purify chemical substances for industrial purposes such as isolating natural esters (perfumes) and producing pure alcohol.[9] The first among them was Jabir ibn Hayyan (Geber), in the 8th century, who is credited with the invention of numerous chemical apparatus and processes that are still in use today. In particular, his alembic was the first still with retorts which could fully purify chemicals, a precursor to the pot still, and its design has served as inspiration for modern micro-scale distillation apparatus such as the Hickman stillhead.[10] The isolation of ethanol (alcohol) as a pure compound through distillation was first achieved by the Arab chemist Al-Kindi (Alkindus).[11] Petroleum was first distilled by the Persian alchemist Muhammad ibn Zakarīya Rāzi (Rhazes) in the 9th century, for producing kerosene,[12] while steam distillation was invented by Avicenna in the early 11th century, for producing essential oils.[13]
As the works of Middle Eastern scribes made their way to India and became a part of Indian alchemy, several texts dedicated to distillation made their way to Indian libraries.[14] Among these was a treatise written by a scholar from Bagdad in 1034 titled Ainu-s-Sana'ah wa' Auna-s-Sana'ah.[14] Scholar Al-Jawbari travelled to India.[15] By the time of the writing of the Ain-e-Akbari, the process of distillation was well known in India.[16]
Distillation was introduced to medieval Europe through Latin translations of Arabic chemical treatises in the 12th century.[17] In 1500, German alchemist Hieronymus Braunschweig published Liber de arte destillandi (The Book of the Art of Distillation)[18] the first book solely dedicated to the subject of distillation, followed in 1512 by a much expanded version. In 1651, John French published The Art of Distillation the first major English compendium of practice, though it has been claimed[19] that much of it derives from Braunschweig's work. This includes diagrams with people in them showing the industrial rather than bench scale of the operation.
As alchemy evolved into the science of chemistry, vessels called retorts became used for distillations. Both alembics and retorts are forms of glassware with long necks pointing to the side at a downward angle which acted as air-cooled condensers to condense the distillate and let it drip downward for collection. Later, copper alembics were invented. Riveted joints were often kept tight by using various mixtures, for instance a dough made of rye flour.[20] These alembics often featured a cooling system around the beak, using cold water for instance, which made the condensation of alcohol more efficient. These were called pot stills. Today, the retorts and pot stills have been largely supplanted by more efficient distillation methods in most industrial processes. However, the pot still is still widely used for the elaboration of some fine alcohols such as cognac, Scotch whisky, tequila and some vodkas. Pot stills made of various materials (wood, clay, stainless steel) are also used by bootleggers in various countries. Small pot stills are also sold for the domestic production[21] of flower water or essential oils.
Early forms of distillation were batch processes using one vaporization and one condensation. Purity was improved by further distillation of the condensate. Greater volumes were processed by simply repeating the distillation. Chemists were reported to carry out as many as 500 to 600 distillations in order to obtain a pure compound[22].
In the early 19th century the basics of modern techniques including pre-heating and reflux were developed, particularly by the French[22], then in 1830 a British Patent was issued to Aeneas Coffey for a whiskey distillation column[23], which worked continuously and may be regarded as the archetype of modern petrochemical units. In 1877, Ernest Solvay was granted a U.S. Patent for a tray column for ammonia distillation[24] and the same and subsequent years saw developments of this theme for oil and spirits.
With the emergence of chemical engineering as a discipline at the end of the 19th century, scientific rather than empirical methods could be applied. The developing petroleum industry in the early 20th century provided the impetus for the development of accurate design methods such as the McCabe-Thiele method and the Fenske equation. The availability of powerful computers has also allowed direct computer simulation of distillation columns.
[edit] Applications of distillation
The application of distillation can roughly be divided in four groups: laboratory scale, industrial distillation, distillation of herbs for perfumery and medicinals (herbal distillate), and food processing. The latter two are distinct from the former two in that the distillation is not used as a true purification method but rather to transfer all volatiles from the source materials to the distillate.
The main difference between laboratory scale distillation and industrial distillation is that laboratory scale distillation is often performed batch-wise, whereas industrial distillation often occurs continuously. In batch distillation, the composition of the source material, the vapors of the distilling compounds and the distillate change during the distillation. In batch distillation, a still is charged (supplied) with a batch of feed mixture, which is then separated into its component fractions which are collected sequentially from most volatile to less volatile, with the bottoms (remaining least or non-volatile fraction) removed at the end. The still can then be recharged and the process repeated.
In continuous distillation, the source materials, vapors, and distillate are kept at a constant composition by carefully replenishing the source material and removing fractions from both vapor and liquid in the system. This results in a better control of the separation process.
[edit] Idealized distillation model
The boiling point of a liquid is the temperature at which the vapor pressure of the liquid equals the pressure in the liquid, enabling bubbles to form without being crushed. A special case is the normal boiling point, where the vapor pressure of the liquid equals the ambient atmospheric pressure.
It is a common misconception that in a liquid mixture at a given pressure, each component boils at the boiling point corresponding to the given pressure and the vapors of each component will collect separately and purely. This, however, does not occur even in an idealized system. Idealized models of distillation are essentially governed by Raoult's law and Dalton's law, and assume that vapor-liquid equilibria are attained.
Raoult's law assumes that a component contributes to the total vapor pressure of the mixture in proportion to its percentage of the mixture and its vapor pressure when pure, or succinctly: partial pressure equals mole fraction multiplied by vapor pressure when pure. If one component changes another component's vapor pressure, or if the volatility of a component is dependent on its percentage in the mixture, the law will fail.
Dalton's law states that the total vapor pressure is the sum of the vapor pressures of each individual component in the mixture. When a multi-component liquid is heated, the vapor pressure of each component will rise, thus causing the total vapor pressure to rise. When the total vapor pressure reaches the pressure surrounding the liquid, boiling occurs and liquid turns to gas throughout the bulk of the liquid. Note that a mixture with a given composition has one boiling point at a given pressure, when the components are mutually soluble.
An implication of one boiling point is that lighter components never cleanly "boil first". At boiling point, all volatile components boil, but for a component, its percentage in the vapor is the same as its percentage of the total vapor pressure. Lighter components have a higher partial pressure and thus are concentrated in the vapor, but heavier volatile components also have a (smaller) partial pressure and necessarily evaporate also, albeit being less concentrated in the vapor. Indeed, batch distillation and fractionation succeed by varying the composition of the mixture. In batch distillation, the batch evaporates, which changes its composition; in fractionation, liquid higher in the fractionation column contains more lights and boils at lower temperatures.
The idealized model is accurate in the case of chemically similar liquids, such as benzene and toluene. In other cases, severe deviations from Raoult's law and Dalton's law are observed, most famously in the mixture of ethanol and water. These compounds, when heated together, form an azeotrope, which is a composition with a boiling point higher or lower than the boiling point of each separate liquid. Virtually all liquids, when mixed and heated, will display azeotropic behaviour. Although there are computational methods that can be used to estimate the behavior of a mixture of arbitrary components, the only way to obtain accurate vapor-liquid equilibrium data is by measurement.
It is not possible to completely purify a mixture of components by distillation, as this would require each component in the mixture to have a zero partial pressure. If ultra-pure products are the goal, then further chemical separation must be applied. When a binary mixture is evaporated and the other component, e.g. a salt, has zero partial pressure for practical purposes, the process is simpler and is called evaporation in engineering.
[edit] Batch distillation
Heating an ideal mixture of two volatile substances A and B (with A having the higher volatility, or lower boiling point) in a batch distillation setup (such as in an apparatus depicted in the opening figure) until the mixture is boiling results in a vapor above the liquid which contains a mixture of A and B. The ratio between A and B in the vapor will be different from the ratio in the liquid: the ratio in the liquid will be determined by how the original mixture was prepared, while the ratio in the vapor will be enriched in the more volatile compound, A (due to Raoult's Law, see above). The vapor goes through the condenser and is removed from the system. This in turn means that the ratio of compounds in the remaining liquid is now different from the initial ratio (i.e. more enriched in B than the starting liquid).
The result is that the ratio in the liquid mixture is changing, becoming richer in component B. This causes the boiling point of the mixture to rise, which in turn results in a rise in the temperature in the vapor, which results in a changing ratio of A : B in the gas phase (as distillation continues, there is an increasing proportion of B in the gas phase). This results in a slowly changing ratio A : B in the distillate.
If the difference in vapor pressure between the two components A and B is large (generally expressed as the difference in boiling points), the mixture in the beginning of the distillation is highly enriched in component A, and when component A has distilled off, the boiling liquid is enriched in component B.
[edit] Continuous distillation
Continuous distillation is an ongoing distillation in which a liquid mixture is continuously (without interruption) fed into the process and separated fractions are removed continuously as output streams as time passes during the operation. Continuous distillation produces at least two output fractions, including at least one volatile distillate fraction, which has boiled and been separately captured as a vapor condensed to a liquid. There is always a bottoms (or residue) fraction, which is the least volatile residue that has not been separately captured as a condensed vapor.
Continuous distillation differs from batch distillation in the respect that concentrations should not change over time. Continuous distillation can be run at a steady state for an arbitrary amount of time. Given a feed of in a specified composition, the main variables that affect the purity of products in continuous distillation are the reflux ratio and the number of theoretical equilibrium stages (practically, the number of trays or the height of packing). Reflux is a flow from the condenser back to the column, which generates a recycle that allows a better separation with a given number of trays. Equilibrium stages are ideal steps where compositions achieve vapor-liquid equilibrium, repeating the separation process and allowing better separation given a reflux ratio. A column with a high reflux ratio may have fewer stages, but it refluxes a large amount of liquid, giving a wide column with a large holdup. Conversely, a column with a low reflux ratio must have a large number of stages, thus requiring a taller column.
Continuous distillation requires building and configuring dedicated equipment. The resulting high investment cost restricts its use to the large scale.
[edit] General improvements
Both batch and continuous distillations can be improved by making use of a fractionating column on top of the distillation flask. The column improves separation by providing a larger surface area for the vapor and condensate to come into contact. This helps it remain at equilibrium for as long as possible. The column can even consist of small subsystems ('trays' or 'dishes') which all contain an enriched, boiling liquid mixture, all with their own vapor-liquid equilibrium.
There are differences between laboratory-scale and industrial-scale fractionating columns, but the principles are the same. Examples of laboratory-scale fractionating columns (in increasing efficacy) include:
- Air condenser
- Vigreux column (usually laboratory scale only)
- Packed column (packed with glass beads, metal pieces, or other chemically inert material)
- Spinning band distillation system
[edit] Laboratory scale distillation
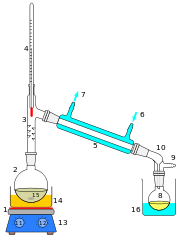
Laboratory scale distillations are almost exclusively run as batch distillations. The device used in distillation, sometimes referred to as a still, consists at a minimum of a reboiler or pot in which the source material is heated, a condenser in which the heated vapour is cooled back to the liquid state, and a receiver in which the concentrated or purified liquid, called the distillate, is collected. Several laboratory scale techniques for distillation exist (see also distillation types).
[edit] Simple distillation
In simple distillation, all the hot vapors produced are immediately channeled into a condenser which cools and condenses the vapors. Therefore, the distillate will not be pure - its composition will be identical to the composition of the vapors at the given temperature and pressure, and can be computed from Raoult's law.
As a result, simple distillation is usually used only to separate liquids whose boiling points differ greatly (rule of thumb is 25 °C),[25] or to separate liquids from involatile solids or oils. For these cases, the vapor pressures of the components are usually sufficiently different that Raoult's law may be neglected due to the insignificant contribution of the less volatile component. In this case, the distillate may be sufficiently pure for its intended purpose.
[edit] Fractional distillation
For many cases, the boiling points of the components in the mixture will be sufficiently close that Raoult's law must be taken into consideration. Therefore, fractional distillation must be used in order to separate the components well by repeated vaporization-condensation cycles within a packed fractionating column. This separation, by successive distillations, is also referred to as rectification [26].
As the solution to be purified is heated, its vapors rise to the fractionating column. As it rises, it cools, condensing on the condenser walls and the surfaces of the packing material. Here, the condensate continues to be heated by the rising hot vapors; it vaporizes once more. However, the composition of the fresh vapors are determined once again by Raoult's law. Each vaporization-condensation cycle (called a theoretical plate) will yield a purer solution of the more volatile component.[27] In reality, each cycle at a given temperature does not occur at exactly the same position in the fractionating column; theoretical plate is thus a concept rather than an accurate description.
More theoretical plates lead to better separations. A spinning band distillation system uses a spinning band of Teflon or metal to force the rising vapors into close contact with the descending condensate, increasing the number of theoretical plates.[28]
[edit] Steam distillation
Like vacuum distillation, steam distillation is a method for distilling compounds which are heat-sensitive. This process involves using bubbling steam through a heated mixture of the raw material. By Raoult's law, some of the target compound will vaporize (in accordance with its partial pressure). The vapor mixture is cooled and condensed, usually yielding a layer of oil and a layer of water.
Steam distillation of various aromatic herbs and flowers can result in two products; an essential oil as well as a watery herbal distillate. The essential oils are often used in perfumery and aromatherapy while the watery distillates have many applications in aromatherapy, food processing and skin care.

[edit] Vacuum distillation
Some compounds have very high boiling points. To boil such compounds, it is often better to lower the pressure at which such compounds are boiled instead of increasing the temperature. Once the pressure is lowered to the vapor pressure of the compound (at the given temperature), boiling and the rest of the distillation process can commence. This technique is referred to as vacuum distillation and it is commonly found in the laboratory in the form of the rotary evaporator.
This technique is also very useful for compounds which boil beyond their decomposition temperature at atmospheric pressure and which would therefore be decomposed by any attempt to boil them under atmospheric pressure.
Molecular distillation is vacuum distillation below the pressure of 0.01 torr.[29] In fact, 0.01 torr is rarefied medium vacuum or only one order of magnitude above high vacuum, where the mean free path of molecules is comparable to the size of the equipment. The gaseous phase no longer exerts significant pressure on the substance to be evaporated, and consequently, rate of evaporation no longer depends on pressure. That is, because the continuum assumptions of fluid dynamics no longer apply, mass transport is governed by molecular dynamics rather than fluid dynamics. Thus, a short path between the hot surface and the cold surface is necessary, typically by suspending a hot plate covered with a film of feed next to a cold plate with a clear line of sight in between. Molecular distillation is used industrially for purification of oils.
[edit] Air-sensitive vacuum distillation
Some compounds have high boiling points as well as being air sensitive. A simple vacuum distillation system as exemplified above can be used, whereby the vacuum is replaced with an inert gas after the distillation is complete. However, this is a less satisfactory system if one desires to collect fractions under a reduced pressure. To do this a "pig" adaptor can be added to the end of the condenser, or for better results or for very air sensitive compounds a Perkin triangle apparatus can be used.
The Perkin triangle, has means via a series of glass or Teflon taps to allows fractions to be isolated from the rest of the still, without the main body of the distillation being removed from either the vacuum or heat source, and thus can remain in a state of reflux. To do this, the sample is first isolated from the vacuum by means of the taps, the vacuum over the sample is then replaced with an inert gas (such as nitrogen or argon) and can then be stoppered and removed. A fresh collection vessel can then be added to the system, evacuated and linked back into the distillation system via the taps to collect a second fraction, and so on, until all fractions have been collected.
[edit] Short path distillation

Short path distillation is a distillation technique that involves the distillate traveling a short distance, often only a few centimeters. A classic example would be a distillation involving the distillate traveling from one glass bulb to another, without the need for a condenser separating the two chambers. This technique is often used for compounds which are unstable at high temperatures. The advantage is that the heating temperature can be considerably lower (at this reduced pressure) than the boiling point of the liquid at standard pressure, and that the distillate only has to travel a short distance before condensing. The Kugelrohr is a kind of a short path distillation apparatus.
[edit] Other types
- The process of reactive distillation involves using the reaction vessel as the still. In this process, the product is usually significantly lower-boiling than its reactants. As the product is formed from the reactants, it is vaporized and removed from the reaction mixture. This technique is an example of a continuous vs. a batch process; advantages include less downtime to charge the reaction vessel with starting material, and less workup.
- Pervaporation is a method for the separation of mixtures of liquids by partial vaporization through a non-porous membrane.
- Extractive distillation is defined as distillation in the presence of a miscible, high boiling, relatively non-volatile component, the solvent, that forms no azeotrope with the other components in the mixture.
- Flash evaporation (or partial evaporation) is the partial vaporization that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through a throttling valve or other throttling device. This process is one of the simplest unit operations, being equivalent to a distillation with only one equilibrium stage.
- Codistillation is distillation which is performed on mixtures in which the two compounds are not miscible.
The unit process of evaporation may also be called "distillation":
- In rotary evaporation a vacuum distillation apparatus is used to remove bulk solvents from a sample. Typically the vacuum is generated by a water aspirator or a membrane pump.
- In a kugelrohr a short path distillation apparatus is typically used (generally in combination with a (high) vacuum) to distill high boiling (> 300 °C) compounds. The apparatus consists of an oven in which the compound to be distilled is placed, a receiving portion which is outside of the oven, and a means of rotating the sample. The vacuum is normally generated by using a high vacuum pump.
Other uses:
- Dry distillation or destructive distillation, despite the name, is not truly distillation, but rather a chemical reaction known as pyrolysis in which solid substances are heated in an inert or reducing atmosphere and any volatile fractions, containing high-boiling liquids and products of pyrolysis, are collected. The destructive distillation of wood to give methanol is the root of its common name - wood alcohol.
- Freeze distillation is an analogous method of purification using freezing instead of evaporation. It is not truly distillation, but a recrystallization where the product is the mother liquor, and does not produce products equivalent to distillation. This process is used in the production of ice beer and ice wine to increase ethanol and sugar content, respectively. Unlike distillation, freeze distillation of ferment concentrates poisonous congeners rather than removing them like distillation.
[edit] Azeotropic distillation
Interactions between the components of the solution create properties unique to the solution, as most processes entail nonideal mixtures, where Raoult's law does not hold. Such interactions can result in a constant-boiling azeotrope which behaves as if it were a pure compound (i.e., boils at a single temperature instead of a range). At an azeotrope, the solution contains the given component in the same proportion as the vapor, so that evaporation does not change the purity, and distillation does not effect separation. For example, ethyl alcohol and water form an azeotrope of 95.6% at 78.1 °C.
If the azeotrope is not considered sufficiently pure for use, there exist some techniques to break the azeotrope to give a pure distillate. This set of techniques are known as azeotropic distillation. Some techniques achieve this by "jumping" over the azeotropic composition (by adding an additional component to create a new azeotrope, or by varying the pressure). Others work by chemically or physically removing or sequestering the impurity. For example, to purify ethanol beyond 95%, a drying agent or a (desiccant such as potassium carbonate) can be added to convert the soluble water into insoluble water of crystallization. Molecular sieves are often used for this purpose as well.
Immiscible liquids, such as water and toluene, easily form azeotropes. Commonly, these azeotropes are referred to as a low boiling azeotrope because the boiling point of the azeotrope is lower than the boiling point of either pure component. The temperature and composition of the azeotrope is easily predicted from the vapor pressure of the pure components, without use of Raoult's law. The azeotrope is easily broken in a distillation set-up by using a liquid-liquid separator ( a decanter ) to separate the two liquid layers that are condensed overhead. Only one of the two liquid layers is refluxed to the distillation set-up.
High boiling azeotropes, such as a 20 weight percent mixture of hydrochloric acid in water, also exist. As implied by the name, the boiling point of the azeotrope is greater than the boiling point of either pure component.
To break azeotropic distillations and cross distillation boundaries, such as in the DeRosier Problem, it is necessary to increase the composition of the light key in the distillate.
[edit] Breaking an azeotrope with unidirectional pressure manipulation
The boiling points of components in an azeotrope overlap to form a band. By exposing an azeotrope to a vacuum or positive pressure, it's possible to bias the boiling point of one component away from the other by exploiting the differing vapour pressure curves of each; the curves may overlap at the azeotropic point, but are unlikely to be remain identical further along the pressure axis either side of the azeotropic point. When the bias is great enough, the two boiling points no longer overlap and so the azeotropic band disappears.
This method can remove the need to add other chemicals to a distillation, but it has two potential drawbacks.
Under negative pressure, power for a vacuum source is needed and the reduced boiling points of the distillates requires that the condenser be run cooler to prevent distillate vapours being lost to the vacuum source. Increased cooling demands will often require additional energy and possibly new equipment or a change of coolant.
Alternatively, if positive pressures are required, standard glassware can not be used, energy must be used for pressurization and there is a higher chance of side reactions occurring in the distillation, such as decomposition, due to the higher temperatures required to effect boiling.
A unidirectional distillation will rely on a pressure change in one direction, either positive or negative.
[edit] Pressure-swing Distillation
Pressure-swing distillation is essentially the same as the unidirectional distillation used to break azeotropic mixtures, but here both positive and negative pressures may be employed.
This has an important impact on the selectivity of the distillation and allows a chemist to optimize a process such that fewer extremes of pressure and temperature are required and less energy is consumed. This is particularly important in commercial applications.
Pressure-swing distillation is employed during the industrial purification of ethyl acetate after its catalytic synthesis from ethanol.
[edit] Industrial distillation
Large scale industrial distillation applications include both batch and continuous fractional, vacuum, azeotropic, extractive, and steam distillation. The most widely used industrial applications of continuous, steady-state fractional distillation are in petroleum refineries, petrochemical and chemical plants and natural gas processing plants.
Industrial distillation[30][26] is typically performed in large, vertical cylindrical columns known as distillation towers or distillation columns with diameters ranging from about 65 centimeters to 16 meters and heights ranging from about 6 meters to 90 meters or more. When the process feed has a diverse composition, as in distilling crude oil, liquid outlets at intervals up the column allow for the withdrawal of different fractions or products having different boiling points or boiling ranges. The "lightest" products (those with the lowest boiling point) exit from the top of the columns and the "heaviest" products (those with the highest boiling point) exit from the bottom of the column and are often called the bottoms.
Large-scale industrial towers use reflux to achieve a more complete separation of products. Reflux refers to the portion of the condensed overhead liquid product from a distillation or fractionation tower that is returned to the upper part of the tower as shown in the schematic diagram of a typical, large-scale industrial distillation tower. Inside the tower, the downflowing reflux liquid provides cooling and condensation of the upflowing vapors thereby increasing the efficacy of the distillation tower. The more reflux is provided for a given number of theoretical plates, the better is the tower's separation of lower boiling materials from higher boiling materials. Alternatively, the more reflux is provided for a given desired separation, the fewer theoretical plates are required.
Such industrial fractionating towers are also used in air separation, producing liquid oxygen, liquid nitrogen, and high purity argon. Distillation of chlorosilanes also enables the production of high-purity silicon for use as a semiconductor.
Design and operation of a distillation tower depends on the feed and desired products. Given a simple, binary component feed, analytical methods such as the McCabe-Thiele method[26][31] or the Fenske equation[26] can be used. For a multi-component feed, simulation models are used both for design and operation. Moreover, the efficiencies of the vapor-liquid contact devices (referred to as "plates" or "trays") used in distillation towers are typically lower than that of a theoretical 100% efficient equilibrium stage. Hence, a distillation tower needs more trays than the number of theoretical vapor-liquid equilibrium stages.
In industrial uses, sometimes a packing material is used in the column instead of trays, especially when low pressure drops across the column are required, as when operating under vacuum.

This packing material can either be random dumped packing (1-3" wide) such as Raschig rings or structured sheet metal. Liquids tend to wet the surface of the packing and the vapors pass across this wetted surface, where mass transfer takes place. Unlike conventional tray distillation in which every tray represents a separate point of vapor-liquid equilibrium, the vapor-liquid equilibrium curve in a packed column is continuous. However, when modeling packed columns, it is useful to compute a number of "theoretical stages" to denote the separation efficiency of the packed column with respect to more traditional trays. Differently shaped packings have different surface areas and void space between packings. Both of these factors affect packing performance.
Another factor in addition to the packing shape and surface area that affects the performance of random or structured packing is the liquid and vapor distribution entering the packed bed. The number of theoretical stages required to make a given separation is calculated using a specific vapor to liquid ratio. If the liquid and vapor are not evenly distributed across the superficial tower area as it enters the packed bed, the liquid to vapor ratio will not be correct in the packed bed and the required separation will not be achieved. The packing will appear to not be working properly. The height equivalent of a theoretical plate (HETP) will be greater than expected. The problem is not the packing itself but the mal-distribution of the fluids entering the packed bed. Liquid mal-distribution is more frequently the problem than vapor. The design of the liquid distributors used to introduce the feed and reflux to a packed bed is critical to making the packing perform to it maximum efficiency. Methods of evaluating the effectiveness of a liquid distributor to evenly distribute the liquid entering a packed bed can be found in references.[33][34] Considerable work as been done on this topic by Fractionation Research, Inc. (commonly known as FRI).[35]
[edit] Distillation in food processing
[edit] Distilled beverages
Carbohydrate-containing plant materials are allowed to ferment, producing a dilute solution of ethanol in the process. Spirits such as whiskey and rum are prepared by distilling these dilute solutions of ethanol. Components other than ethanol, including water, esters, and other alcohols, are collected in the condensate, which account for the flavor of the beverage.
[edit] References
- ^ Martin Levey (1956). "Babylonian Chemistry: A Study of Arabic and Second Millennium B.C. Perfumery", Osiris 12, p. 376-389.
- ^ a b Allchin 1979
- ^ a b Colin Archibald Russell (2000). Chemistry, Society and Environment: A New History of the British Chemical Industry. Royal Society of Chemistry. pp. p.69. ISBN 0854045996.
- ^ Edgar Ashworth Underwood. Science, Medicine, and History: Essays on the Evolution of Scientific Thought and Medical. Oxford University Press. pp. 251.
- ^ a b Charles Simmonds (1919). Alcohol: With Chapters on Methyl Alcohol, Fusel Oil, and Spirituous Beverages. Macmillan and Co. Ltd. pp. 6.
- ^ Distillation - LoveToKnow 1911
- ^ Biology, Joan Solomon, Pat O'Brien, Peter Horsfall, Nelson Thornes, p.41
- ^ Forbes 1970: 53-54
- ^ Robert Briffault (1938), The Making of Humanity, p. 195:
"Chemistry, the rudiments of which arose in the processes employed by Egyptian metallurgists and jewellers combining metals into various alloys and 'tinting' them to resemble gold processes long preserved as a secret monopoly of the priestly colleges, and clad in the usual mystic formulas, developed in the hands of the Arabs into a widespread, organized passion for research which led them to the invention of pure distillation, sublimation, filtration, to the discovery of alcohol, of nitric and sulfuric acids (the only acid known to the ancients was vinegar), of the alkalis, of the salts of mercury, of antimony and bismuth, and laid the basis of all subsequent chemistry and physical research."
- ^ Microscale Laboratory Techniques - Distillation from McMaster University
- ^ Hassan, Ahmad Y. "Alcohol and the Distillation of Wine in Arabic Sources". History of Science and Technology in Islam. http://www.history-science-technology.com/Notes/Notes%207.htm. Retrieved on 2008-03-29.
- ^ Kasem Ajram (1992). Miracle of Islamic Science. Knowledge House Publishers. Appendix B. ISBN 0911119434.
- ^ A. Wolf, G. A. Bray, B. M. Popkin (2007). "A short history of beverages and how our body treats them". Obesity Reviews 9: 151. doi:.
- ^ a b Forbes 1970: 42
- ^ Forbes 1970: 45
- ^ Forbes 1970: 54
- ^ Hassan, Ahmad Y. "Technology Transfer in the Chemical Industries". History of Science and Technology in Islam. http://www.history-science-technology.com/Articles/articles%2072.htm. Retrieved on 2008-03-29.
- ^ Magnum Opus Hermetic Sourceworks Series
- ^ Industrial Engineering Chemistry (1936) page 677
- ^ Sealing Technique, accessed 16 November 2006.
- ^ Traditional Alembic Pot Still, accessed 16 November 2006.
- ^ a b D. F. Othmer (1982) Distillation - Some Steps in its Development, in W. F. Furter (ed) A Century of Chemical Engineering ISBN 0-306-40895-3
- ^ A. Coffey British Patent 5974, 5 August 1830
- ^ US Patent 198699 Improvement in the Ammonia-Soda Manufacture
- ^ ST07 Separation of liquid - liquid mixtures (solutions), DIDAC by IUPAC
- ^ a b c d Perry, Robert H. and Green, Don W. (1984). Perry's Chemical Engineers' Handbook (6th Edition ed.). McGraw-Hill. ISBN 0-07-049479-7.
- ^ Fractional Distillation
- ^ Spinning Band Distillation at B/R Instrument Corporation (accessed 8 September 2006)
- ^ Vogel's 5th ed.
- ^ Kister, Henry Z. (1992). Distillation Design (1st Edition ed.). McGraw-Hill. ISBN 0-07-034909-6.
- ^ Seader, J. D., and Henley, Ernest J.. Separation Process Principles. New York: Wiley. ISBN 0-471-58626-9.
- ^ Energy Institute website page
- ^ Random Packing, Vapor and Liquid Distribution: Liquid and gas distribution in commercial packed towers, Moore, F., Rukovena, F., Chemical Plants & Processing, Edition Europe, August 1987, p. 11-15
- ^ Structured Packing, Liquid Distribution: A new method to assess liquid distributor quality, Spiegel, L., Chemical Engineering and Processing 45 (2006), p. 1011-1017
- ^ Packed Tower Distributors: Commercial Scale Experiments That Provide Insight on Packed Tower Distributors, Kunesh, J. G., Lahm, L., Yanagi, T., Ind. Eng. Chem. Res., 1987, vol. 26, p. 1845-1850 FRI (click on "Available Materials" and scroll to "Staff Publications")
[edit] Further reading
- Forbes, R. J. (1970). A Short History of the Art of Distillation from the Beginnings up to the Death of Cellier Blumenthal. BRILL. ISBN 9004006176.
- Needham, Joseph (1954). Science and Civilisation in China (vol. 4) Cambridge University Press: ISBN 052108573X
- Allchin, F. R. (Mar., 1979). India: The Ancient Home of Distillation?. Man, New Series, Vol. 14, No. 1 , pp. 55-63. Royal Anthropological Institute of Great Britain and Ireland.
[edit] Gallery
 |
Chemistry on its beginnings used retorts as laboratory equipment exclusively for distillation processes. |
 |
A simple set-up to distill dry and oxygen-free toluene. |
 |
Diagram of an industrial-scale vacuum distillation column as commonly used in oil refineries |
 |
A rotary evaporator is able to distill solvents more quickly at lower temperatures through the use of a vacuum. |
 |
Distillation using semi-microscale apparatus. The jointless design eliminates the need to fit pieces together. The pear-shaped flask allows the last drop of residue to be removed, compared with a similarly-sized round-bottom flask The small holdup volume prevents losses. A pig is used to channel the various distillates into three receiving flasks. If necessary the distillation can be carried out under vacuum using the vacuum adapter at the pig. |
[edit] External links
| Look up distillation in Wiktionary, the free dictionary. |
| Wikimedia Commons has media related to: Distillation |
- Alcohol distillation
- Case Study: Petroleum Distillation
- "Binary Vapor-Liquid Equilibrium Data" (searchable database). Chemical Engineering Research Information Center. http://www.cheric.org/research/kdb/hcvle/hcvle.php. Retrieved on 5 May 2007.
|
||||||||||||||||
|
||||||||||||||||