Chagas disease
From Wikipedia, the free encyclopedia
| Chagas disease Classification and external resources |
|
| An acute Chagas disease infection with swelling of the right eye (Romaña's sign). Source: CDC. | |
| ICD-10 | B57. |
| ICD-9 | 086 |
| DiseasesDB | 13415 |
| MedlinePlus | 001372 |
| eMedicine | med/327 |
| MeSH | D014355 |
Chagas disease (Portuguese: doença de Chagas, Spanish: enfermedad de Chagas-Mazza, mal de Chagas in both languages; also called American trypanosomiasis) is a tropical parasitic disease caused by the flagellate protozoan Trypanosoma cruzi. T. cruzi is commonly transmitted to humans and other mammals by an insect vector, the blood-sucking assassin bugs of the subfamily Triatominae (family Reduviidae) most commonly species belonging to the Triatoma, Rhodnius, and Panstrongylus genera. The disease may also be spread through blood transfusion and organ transplantation, ingestion of food contaminated with parasites, and from a mother to her fetus.
The symptoms of Chagas disease vary over the course of an infection. In the early, acute stage, symptoms are mild and usually produce no more than local swelling at the site of infection. As the disease progresses, over the course of many years, serious chronic symptoms can appear, such as heart disease and malformation of the intestines. If untreated, the chronic disease is often fatal. Current drug treatments are generally unsatisfactory; available medications are highly toxic and often ineffective, particularly those used to treat the chronic stage of the disease. Secondary achalasia may arise from Chagas's disease.
Chagas disease occurs exclusively in the Americas, particularly in poor, rural areas of Mexico, Central America, and South America; very rarely, the disease has originated in the Southern United States. The insects that spread the disease are known by various local names, including vinchuca in Argentina, Bolivia and Paraguay, barbeiro (the barber) in Brazil, Pito in Colombia, chipo, chupança, chinchorro, and "the kissing bug". It is estimated that as many as 8 to 11 million people in Mexico, Central America, and South America have Chagas disease, most of whom do not know they are infected. Large-scale population movements from rural to urban areas of Latin America and to other regions of the world have increased the geographic distribution of Chagas disease. Control strategies have mostly focused on eliminating the triatomine vector and preventing transmission from other sources.[1]
Contents |
[edit] History
The disease was named after the Brazilian physician and infectologist Carlos Chagas, who first described it in 1909,[2][3] but the disease was not seen as a major public health problem in humans until the 1960s (the outbreak of Chagas disease in Brazil in the 1920s went widely ignored[4]). He discovered that the intestines of Triatomidae harbored a flagellate protozoan, a new species of the Trypanosoma genus, and was able to prove experimentally that it could be transmitted to marmoset monkeys that were bitten by the infected bug. Later studies showed that squirrel monkeys were also vulnerable to infection.[5]
Chagas named the pathogenic parasite Trypanosoma cruzi[2] and later that year as Schizotrypanum cruzi,[6] both honoring Oswaldo Cruz, the noted Brazilian physician and epidemiologist who fought successfully epidemics of yellow fever, smallpox, and bubonic plague in Rio de Janeiro and other cities in the beginning of the 20th century. Chagas’ work is unique in the history of medicine because he was the only researcher so far to describe solely and completely a new infectious disease: its pathogen, vector, host, clinical manifestations, and epidemiology.
Nevertheless, he believed (falsely) until 1925 that the main infection route is by the bite of the insect—and not by its feces, as was proposed by his colleague Emile Brumpt in 1915 and assured by Silveira Dias in 1932, Cardoso in 1938, and Brumpt himself in 1939. Chagas was also the first to unknowingly discover and illustrate the parasitic fungal genus Pneumocystis, later infamously to be linked to PCP (Pneumocystis pneumonia in AIDS victims).[3] Confusion between the two pathogens' life-cycles led him to briefly recognize his genus Schizotrypanum, but following the description of Pneumocystis by others as an independent genus, Chagas returned to the use of the name Trypanosoma cruzi.
In Argentina the disease is called Mal de Chagas-Mazza[7], in honor of Salvador Mazza, the Argentine doctor who in 1926 began investigating the disease and over the years became the principal researcher of this disease in the country. The importance of the work of Salvador Mazza lay in pointing out that the disease was an important issue and to preach in the faculties of Medicine. However, this would only be widely accepted since the 1960s, along with the great impact of the disease to public health.
It has been hypothesized that Charles Darwin might have suffered from Chagas disease as a result of a bite of the so-called Great Black Bug of the Pampas (vinchuca) (see Charles Darwin's illness). The episode was reported by Darwin in his diaries of the Voyage of the Beagle as occurring in March 1835 to the east of the Andes near Mendoza. Darwin was young and generally in good health, though six months previously he had been ill for a month near Valparaiso, but in 1837, almost a year after he returned to England, he began to suffer intermittently from a strange group of symptoms, becoming incapacitated for much of the rest of his life. Attempts to test Darwin's remains at the Westminster Abbey by using modern PCR techniques were met with a refusal by the Abbey's curator.[8]
[edit] Transmission
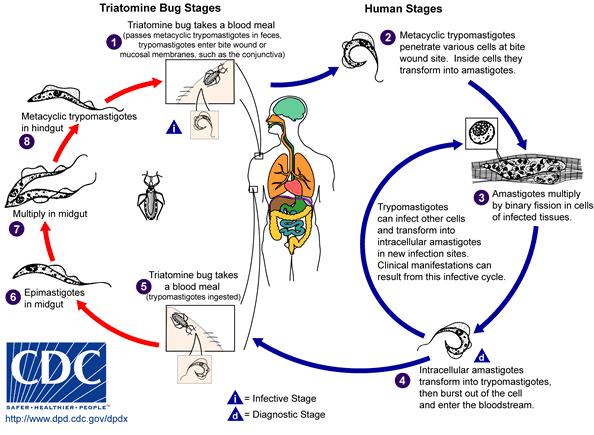
In Chagas-endemic areas, the main mode of transmission is through a insect vector called a triatomine bug.[1] A triatomine becomes infected with T. cruzi by feeding on the blood of an infected person or animal. During the day, triatomine hide in crevices in the walls and roofs. The bugs emerge at night, when the inhabitants are sleeping. Because they tend to feed on people’s faces, triatomine bugs are also known as “kissing bugs.” After they bite and ingest blood, they defecate on the person. Triatomine pass T. cruzi parasites (called trypomastigotes) in feces left near the site of the bite wound. Scratching the site of the bite causes the trypomastigotes to enter the host through the wound, or through intact mucous membranes, such as the conjunctiva. Once inside the host the trypomastigotes invade cells, where they differentiate into intracellular amastigotes. The amastigotes multiply by binary fission and differentiate into trypomastigotes, which are then released into the bloodstream. This cycle is repeated in each newly infected cell. Replication resumes only when the parasites enter another cell or are ingested by another vector.[1]
T. cruzi can also be transmitted through blood transfusions, organ transplantation, breast milk,[9] and by accidental laboratory exposure. Chagas disease can also be spread congenitally (from a pregnant woman to her baby) through the placenta, and accounts for approximately 13% of stillborn deaths in parts of Brazil.[10]
In 1991 farm workers in the state of Paraíba, Brazil, were infected by eating contaminated food; transmission has also occurred via contaminated açaí palm fruit juice and sugar cane juice.[11][12][13] Despite many warnings in the press and by health authorities, this source of infection continues unabated.[14]
[edit] Clinical manifestations
The human disease occurs in two stages: an acute stage, which occurs shortly after an initial infection, and a chronic stage that develops over many years.

The acute phase lasts for the first few weeks or months of infection. It usually occurs unnoticed because it is symptom free or exhibits only mild symptoms and signs that are not unique to Chagas disease. The symptoms noted by the patient can include fever, fatigue, body aches, headache, rash, loss of appetite, diarrhea, and vomiting. The signs on physical examination can include mild enlargement of the liver or spleen, swollen glands, and local swelling (a chagoma) where the parasite entered the body. The most recognized marker of acute Chagas disease is called Romaña's sign, which includes swelling of the eyelids on the side of the face near the bite wound or where the bug feces were deposited or accidentally rubbed into the eye. Even if symptoms develop during the acute phase, they usually resolve spontaneously within 3–8 weeks in approximately 90% of individuals.[15] Although the symptoms resolve, the infection, if untreated, persists. Rarely, young children (<5%), or adults die from severe inflammation/infection of the heart muscle (myocarditis) or brain (meningoencephalitis).[16] The acute phase also can be severe in people with weakened immune systems.[1] In about 10% of infections the symptoms do not completely resolve and result in a so-called chronic latent or indeterminate asymptomatic phase of the disease.
Several years or even decades after initial infection, an estimated 30% of infected people will develop medical problems from Chagas disease over the course of their lives. The symptomatic chronic stage affects the nervous system, digestive system and heart. About two thirds of people with chronic symptoms have cardiac damage, including cardiomyopathy, which causes heart rhythm abnormalities and may result in sudden death. About one third of patients go on to develop digestive system damage, resulting in dilation of the digestive tract (megacolon and megaesophagus), accompanied by severe weight loss. Swallowing difficulties may be the first symptom of digestive disturbances and may lead to malnutrition. Twenty to fifty percent of individuals with intestinal involvement also exhibit cardiac involvement.[15] A small percentage of individuals develop various neurological disorders, including dementia. The clinical manifestations of Chagas disease are due to cell death in the target tissues that occurs during the infective cycle, by sequentially inducing an inflammatory response, cellular lesions, and fibrosis. For example, intracellular amastigotes destroy the intramural neurons of the autonomic nervous system in the intestine and heart, leading to megaintestine and heart aneurysms, respectively. If left untreated, Chagas disease can be fatal, in most cases due to heart muscle damage.[15]
[edit] Diagnosis
The presence of T. cruzi is diagnostic of Chagas disease. It can be detected by Microscopic examination of fresh anti-coagulated blood, or its buffy coat, for motile parasites; or by preparation of thin and thick blood smears stained with Giemsa, for direct visualization of parasites. Microscopically, T. cruzi can be confused with Trypanosoma rangeli, which is not known to be pathogenic in humans. Isolation of T. cruzi can occur by inoculation into mice, by culture in specialized media (e.g., NNN, LIT); and by xenodiagnosis,[17] where uninfected Reduviidae bugs are fed on the patient's blood, and their gut contents examined for parasites.[15]
Various immunoassays for T. cruzi are available and can be used to distinguish among strains (zymodemes of T.cruzi with divergent pathogenicities). These tests include: detecting complement fixation, indirect hemagglutination, indirect fluorescence assays, radioimmunoassays, and ELISA. Alternatively diagnosis and strain identification can be made using polymerase chain reaction (PCR).[15]
[edit] Treatment
There are two approaches to treating Chagas disease, antiparasitic treatment, to kill the parasite; and symptomatic treatment, to manage the symptoms and signs of infection.
Antiparasitic treatment is most effective early in the course of infection but is not limited to cases in the acute phase. Drugs of choice include azole or nitro derivatives such as benznidazole[18] or nifurtimox. However, resistance to these drugs has been reported.[19] Moreover, a 10-year study of chronic administration of antiparasitic drugs in Brazil has revealed that current drug treatment regimens do not fully remove parasitemia.[20]
In the chronic stage, treatment involves managing the clinical manifestations of the disease. For example, pacemakers and medications for irregular heartbeats may be life saving for some patients with chronic cardiac disease, while surgery may be required for megaintestine. The disease cannot be cured in this phase, however. Chronic heart disease caused by Chagas disease is now a common reason for heart transplantation surgery. Until recently, however, Chagas disease was considered a contraindication for the procedure, since the heart damage could recur as the parasite was expected to seize the opportunity provided by the immunosuppression that follows surgery.[21] It was noted that survival rates in Chagas patients could be significantly improved by using lower dosages of the immunosuppressant drug cyclosporin. Recently, direct stem cell therapy of the heart muscle using bone marrow cell transplantation has been shown to dramatically reduce risks of heart failure in Chagas patients.[22]
Several experimental treatments have shown promise in animal models. These include inhibitors of oxidosqualene cyclase and squalene synthase,[23][24] cysteine protease inhibitors,[23][25] dermaseptins collected from frogs in the genus Phyllomedusa (P. oreades and P. distincta),[26] the sesquiterpene lactone dehydroleucodine (DhL) which affects the growth of cultured epimastigotes of Trypanosoma cruzi,[27] inhibitors of purine uptake,[23] and inhibitors of enzymes involved in trypanothione metabolism.[28] It is hoped that new drug targets may be revealed following the sequencing of the T. cruzi genome.[29]
[edit] Prevention

There is currently no vaccine against Chagas disease[30] and prevention is generally focused on fighting the vector Triatoma by using sprays and paints containing insecticides (synthetic pyrethroids), and improving housing and sanitary conditions in rural areas.[31] For urban dwellers, spending vacations and camping out in the wilderness or sleeping at hostels or mud houses in endemic areas can be dangerous; a mosquito net is recommended.
A number of potential vaccines are currently being tested. Vaccination with Trypanosoma rangeli has produced positive results in animal models.[32] More recently, the potential of DNA vaccines for immunotherapy of acute and chronic Chagas disease is being tested by several research groups.[33]
Blood transfusion is the second most common transmission route of Chagas disease in many Latin American countries. In 1993 a series of serologic surveys, looking for antibodies against T. cruzi in blood donors, revealed that the probability of receiving a potentially infected transfusion unit in each country varied from 1.4% to 18% in Argentina, Brazil, and Chile, and can be up to 48% in Bolivia. Suggesting that in this region there is a high risk of transfusion acquired Chagas; much higher than the risks reported for other infections acquired through blood such as hepatitis (0.1%) and AIDS (0.1%) in the same region.[34] The likelihood of being infected when receiving an infected transfusion unit were assumed to be 20% for T. cruzi.[35] Vector control efforts have, however, led to a reduction in infection rates; the prevalence of T. cruzi infection in the Brazilian blood bank system was 0.96% in 1996, down from 2% in the 1970s.[36] In most countries where Chagas disease is endemic, testing of blood donors is already mandatory, since this can be an important route of transmission.[37] The United States FDA has recently licensed a test for antibodies against T. cruzi for use on blood donors but has not yet mandated its use.[38] The American Association of Blood Banks recommends that past recipients of blood components from donors found to be infected be notified and themselves tested.[39] In the past, donated blood was mixed with 0.25 g/L of gentian violet, which kills T. cruzi parasites.[40] The antifungal agent amphotericin B has been proposed as a second-line treatment, but the high cost and relatively high toxicity of the drug have limited its use.[41]
[edit] Distribution
Chagas disease affects 16–18 million people as of 2008, with some 100 million (25% of the Latin American population) at risk of acquiring the disease,[42] killing around 20,000 people annually.[15]
The disease is present in 18 countries on the American continent, ranging from the southern United States to southern Argentina.[1] Chagas exists in two different ecological zones. In the Southern Cone region the main vector lives in and around human homes. In Central America and Mexico the main vector species lives both inside dwellings and in uninhabited areas. In both zones Chagas occurs almost exclusively in rural areas, where triatomine breed and feed on the over 150 species from 24 families of domestic and wild mammals, as well as humans, that are the natural reservoirs of T.cruzi.[37] Although Triatominae bugs feed on birds, they appear to be immune against infection and therefore are not considered to be a T. cruzi reservoir. Even when colonies of insects are eradicated from a house and surrounding domestic animal shelters, they can re-emerge from plants or animals that are part of the ancient, sylvatic (referring to wild animals) infection cycle. This is especially likely in zones with mixed open savannah, with clumps of trees interspersed by human habitation.[43]

Dense vegetation (such as that of tropical rainforests) and urban habitats are not ideal for the establishment of the human transmission cycle. However, in regions where the sylvatic habitat and its fauna are thinned by economical exploitation and human habitation, such as in newly deforested areas, piassava palm culture areas, and some parts of the Amazon region, a human transmission cycle may develop as the insects search for new food sources.[44]
The primary wildlife reservoirs for Trypanosoma cruzi in the United States include opossums, raccoons, armadillos, squirrels, woodrats and mice.[45] Opossums are particularly important as reservoirs because the parasite can complete its life cycle in the anal glands of the animal without having to re-enter the insect vector.[45] Recorded prevalence of the disease in opossums in the U.S. ranges from 8.3%[45] up to 37.5%.[46] Studies on raccoons in the Southeast have yielded infection rates ranging from 47%[47] to as low as 15.5%.[45] Armadillo prevalence studies have been described in Louisiana and range from a low of 1.1%[46] up to 28.8%.[48] Additionally small rodents including squirrels, mice and rats are important in the sylvatic transmission cycle because of their importance as bloodmeal sources for the insect vectors. A Texas study revealed 17.3% percent T. cruzi prevalence in 75 specimens comprised of four separate small rodent species.[49]
Chronic Chagas disease remains a major health problem in many Latin American countries, despite the effectiveness of hygienic and preventive measures, such as eliminating the transmitting insects, which have reduced to zero new infections in at least two countries of the region. With increased population movements, however, the possibility of transmission by blood transfusion has become more substantial in the United States.[50] Approximately 500,000 infected people live in the United States, which is likely the result of immigration from Latin American countries.[51]
Several landmarks have been achieved in the fight against Chagas disease in Latin America including a reduction by 72% of the incidence of human infection in children and young adults in the countries of the Southern Cone Initiative, and at least two countries (Uruguay, in 1997, and Chile, in 1999) have been certified free of vectorial and transfusional transmission.[52][53] In Argentina vectorial transmission has been interrupted in 13 of the 19 endemic provinces.[53] Brazil has also been certified free of T. infestans transmission,[54] although other vectors, particularly T. brasiliensis and T. pseudomaculata, account for most transmission in the Northeast Region.[55]
Some stepstones of vector control:
- A yeast trap has been tested for monitoring infestations of certain species of triatomine bugs (Triatoma sordida, Triatoma brasiliensis, Triatoma pseudomaculata, and Panstrongylus megistus).[56]
- Promising results were gained with the treatment of vector habitats with the fungus Beauveria bassiana.[57]
- Targeting the symbionts of Triatominae through paratransgenesis.[58]
[edit] See also
[edit] References
- ^ a b c d e "DPDx – Trypanosomiasis, American. Fact Sheet". Centers for Disease Control (CDC). http://www.dpd.cdc.gov/dpdx/HTML/TrypanosomiasisAmerican.htm. Retrieved on 2008-09-11.
- ^ a b Chagas C (1909). "Neue Trypanosomen". Vorläufige Mitteilung Arch Schiff Tropenhyg 13: 120–2.
- ^ a b Redhead SA, Cushion MT, Frenkel JK, Stringer JR (2006). "Pneumocystis and Trypanosoma cruzi: nomenclature and typifications". J Eukaryot Microbiol 53 (1): 2–11. doi:. PMID 16441572.
- ^ Coutinho M (June 1999). "Review of Historical Aspects of American Trypanosomiasis (Chagas' Disease) by Matthias Perleth" (fee required). Isis 90 (2): 397. doi:. http://links.jstor.org/sici?sici=0021-1753%28199906%2990%3A2%3C397%3AHAOAT%28%3E2.0.CO%3B2-H.
- ^ Hulsebos LH, Choromanski L, Kuhn RE (1989). "The effect of interleukin-2 on parasitemia and myocarditis in experimental Chagas' disease". J Protozool 36 (3): 293–8. PMID 2499678.
- ^ Chagas C (1909). "Nova tripanozomiase humana: Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem [New human trypanosomiasis. Studies about the morphology and life-cycle of Schizotripanum cruzi, etiological agent of a new morbid entity of man]". Mem Inst Oswaldo Cruz 1 (2): 159–218.
- ^ http://www.drwebsa.com.ar/alcha/hist4.htm Mal de Chagas-Mazza
- ^ Adler D (1989). "Darwin's Illness". Isr J Med Sci 25 (4): 218–21. PMID 2496051.
- ^ Santos Ferreira C, Amato Neto V, Gakiya E, Bezerra RC, Alarcón RS (2003). "Microwave treatment of human milk to prevent transmission of Chagas disease". Rev Inst Med Trop São Paulo 45 (1): 41–2. PMID 12751321.
- ^ Hudson L, Turner MJ (November 1984). "Immunological consequences of infection and vaccination in South American trypanosomiasis [and discussion]". Philos Trans R Soc Lond, B, Biol Sci 307 (1131): 51–61. doi:. PMID 6151688. http://www.jstor.org/pss/2990154. Retrieved February 22, 2007 through JSTOR.
- ^ "Chagas’ disease (American trypanosomiasis) in southern Brazil" (PDF). CDR Weekly (United Kingdom Health Protection Agency) 15 (13). April 2005. http://www.hpa.org.uk/CDR/archives/2005/cdr1305.pdf. Retrieved on 2007-11-26.
- ^ Shikanai-Yasuda MA, Marcondes CB, Guedes LA, et al (1991). "Possible oral transmission of acute Chagas' disease in Brazil". Rev Inst Med Trop São Paulo 33 (5): 351–7. PMID 1844961.
- ^ da Silva Valente SA, de Costa Valente V, Neto HF (1999). "Considerations on the epidemiology and transmission of Chagas disease in the Brazilian Amazon". Mem Inst Oswaldo Cruz 94 Suppl 1: 395–8. PMID 10677763. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0074-0276(99)09400077&lng=en&nrm=iso&tlng=en.
- ^ Reis, T (August 18, 2007). "Açaí faz 1 vítima de Chagas a cada 4 dias na Amazônia" (in Portuguese). Folha de São Paulo. http://www1.folha.uol.com.br/folha/cotidiano/ult95u321060.shtml. Retrieved on 2008-08-29.
- ^ a b c d e f Carlier Y (27 February 2003). Chagas Disease (American Trypanosomiasis). eMedicine. Retrieved on 11 September 2008.
- ^ Guimarães FN, da Silva NN, Clausell DT, de Mello AL, Rapone T, Snell T, Rodrigues N (1968). "Um surto epidêmico de doença de Chagas de provável transmissão digestiva, ocorrido em Teutonia (Estrêla - Rio Grande Do Sul)". Hospital (Rio J) 73 (6): 1767–804. http://en.calameo.com/read/00000948401a1fdb85f76.
- ^ Brumpt E (1914). "Le xénodiagnostic. Application au diagnostic de quelques infections parasitaires et en particulier à la trypanosomose de Chagas" (PDF). Bull Soc Pathol Exot 7 (10): 706–10. http://www.pathexo.fr/documents/articles-bull/1914/1914n10/T7-10-706.pdf.
- ^ Garcia S, Ramos CO, Senra JF, et al (April 2005). "Treatment with benznidazole during the chronic phase of experimental Chagas' disease decreases cardiac alterations". Antimicrob Agents Chemother 49 (4): 1521–8. doi:. PMID 15793134. PMC: 1068607. http://aac.asm.org/cgi/pmidlookup?view=long&pmid=15793134.
- ^ Buckner FS, Wilson AJ, White TC, Van Voorhis WC (December 1998). "Induction of resistance to azole drugs in Trypanosoma cruzi". Antimicrob Agents Chemother 42 (12): 3245–50. PMID 9835521. PMC: 106029. http://aac.asm.org/cgi/pmidlookup?view=long&pmid=9835521.
- ^ Lauria-Pires L, Braga MS, Vexenat AC, et al (2000). "Progressive chronic Chagas heart disease ten years after treatment with anti-Trypanosoma cruzi nitroderivatives". Am J Trop Med Hyg 63 (3–4): 111–8. PMID 11388500. http://www.ajtmh.org/cgi/pmidlookup?view=long&pmid=11388500.
- ^ Bocchi EA, Bellotti G, Mocelin AO, et al (June 1996). "Heart transplantation for chronic Chagas' heart disease". Ann Thorac Surg 61 (6): 1727–33. doi:. PMID 8651775. http://linkinghub.elsevier.com/retrieve/pii/0003-4975(96)00141-5.
- ^ Vilas-Boas F, Feitosa GS, Soares MB, et al (August 2006). "[Early results of bone marrow cell transplantation to the myocardium of patients with heart failure due to Chagas disease]" (in Portuguese). Arq Bras Cardiol 87 (2): 159–66. PMID 16951834. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0066-782X2006001500014&lng=en&nrm=iso&tlng=en.
- ^ a b c Scientific Working Group on Chagas Disease [April 2005] (July 2007). Guhl F, Lazdins-Helds JK (eds.): Reporte del grupo de trabajo científico sobre la enfermedad de Chagas (in Spanish). Geneva: WHO. Retrieved on August 29, 2008. Executive summary in English
- ^ Urbina JA, Concepcion JL, Rangel S, Visbal G, Lira R (2002). "Squalene synthase as a chemotherapeutic target in Trypanosoma cruzi and Leishmania mexicana". Mol Biochem Parasitol 125 (1–2): 35–45. PMID 12467972. http://linkinghub.elsevier.com/retrieve/pii/S0166685102002062.
- ^ Engel JC, Doyle PS, Hsieh I, McKerrow JH (August 1998). "Cysteine protease inhibitors cure an experimental Trypanosoma cruzi infection". J Exp Med 188 (4): 725–34. PMID 9705954. PMC: 2213346. http://www.jem.org/cgi/pmidlookup?view=long&pmid=9705954.
- ^ Brand GD, Leite JR, Silva LP, et al (December 2002). "Dermaseptins from Phyllomedusa oreades and Phyllomedusa distincta. Anti-Trypanosoma cruzi activity without cytotoxicity to mammalian cells". J Biol Chem 277 (51): 49332–40. doi:. PMID 12379643. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=12379643.
- ^ Brengio SD, Belmonte SA, Guerreiro E, Giordano OS, Pietrobon EO, Sosa MA (April 2000). "The sesquiterpene lactone dehydroleucodine (DhL) affects the growth of cultured epimastigotes of Trypanosoma cruzi". J Parasitol 86 (2): 407–12. PMID 10780563.
- ^ Fairlamb AH, Cerami A (1992). "Metabolism and functions of trypanothione in the Kinetoplastida". Annu Rev Microbiol 46: 695–729. doi:. PMID 1444271. http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.mi.46.100192.003403?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dncbi.nlm.nih.gov.
- ^ El-Sayed NM, Myler PJ, Bartholomeu DC, et al (July 2005). "The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease". Science 309 (5733): 409–15. doi:. PMID 16020725. http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=16020725.
- ^ "A killer that preys on the poor: Chagas disease" (pdf). Médecins Sans Frontières: Activity Report 2003/2004. http://www.msf.org/source/actrep/2004/pdf/62-63.pdf. Retrieved on 2008-08-29.
- ^ Eduardo N. Zerba (1999). "Susceptibility and resistance to insecticides of Chagas disease vectors" (PDF). Medicina (Buenos Aires) 59 (Suppl 2): 41–6. http://www.medicinabuenosaires.com/revistas/vol59-99/supl2/v59_s2_41_46.pdf.
- ^ Basso B, Moretti E, Fretes R (June 2008). "Vaccination with epimastigotes of different strains of Trypanosoma rangeli protects mice against Trypanosoma cruzi infection". Mem Inst Oswaldo Cruz 103 (4): 370–4. PMID 18660992. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0074-02762008000400010&lng=en&nrm=iso&tlng=en.
- ^ Dumonteil E, Escobedo-Ortegon J, Reyes-Rodriguez N, Arjona-Torres A, Ramirez-Sierra M (2004). "Immunotherapy of Trypanosoma cruzi infection with DNA vaccines in mice". Infect Immun 72 (1): 46–53. doi:. PMID 14688079.
- ^ Sánchez-Guillén MC, Barnabé C, Guégan JF, et al (October 2002). "High prevalence anti-Trypanosoma cruzi antibodies, among blood donors in the State of Puebla, a non-endemic area of Mexico". Mem Inst Oswaldo Cruz 97 (7): 947–52. PMID 12471419. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0074-02762002000700004&lng=en&nrm=iso&tlng=en.
- ^ Schmunis GA (1999). "Prevention of transfusional Trypanosoma cruzi infection in Latin America". Mem Inst Oswaldo Cruz 94 Suppl 1: 93–101. PMID 10677696. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0074-02761999000700010&lng=en&nrm=iso. Retrieved on 2008-09-01.
- ^ Vinhaes, MC (November 2001). "VIII. Os programas nacionais de controle na fase avancada de controle e os novos desafios estratégicos, políticos e epidemiológicos" (PDF). Grupo de Trabajo OPS para Consulta en Planificación, Operativa, Estrategia y Evaluación de Etapas Avanzadas del Control Antivectorial en Enfermedad de Chagas, Pan American Health Organization.
- ^ a b Morel CM, Lazdins J (October 2003). "Chagas disease". Nat Rev Microbiol 1 (1): 14–5. doi:. PMID 15040175.
- ^ "Blood donor screening for Chagas disease—United States, 2006–2007". MMWR Morb Mortal Wkly Rep 56 (7): 141–3. February 2007. PMID 17318113. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5607a2.htm.
- ^ Gregory KR (September 12, 2002). "Chagas' Disease". American Association of Blood Banks. http://www.aabb.org/Content/Members_Area/Members_Area_Regulatory/Donor_Testing/chagbpac091202.htm. Retrieved on 2008-08-27.
- ^ Docampo R, Moreno SN, Muniz RP, Cruz FS, Mason RP (June 1983). "Light-enhanced free radical formation and trypanocidal action of gentian violet (crystal violet)". Science 220 (4603): 1292–5. doi:. PMID 6304876. http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=6304876.
- ^ Cruz FS, Marr JJ, Berens RL (September 1980). "Prevention of transfusion-induced Chagas' disease by amphotericin B". Am J Trop Med Hyg 29 (5): 761–5. PMID 6776830. http://www.ajtmh.org/cgi/pmidlookup?view=long&pmid=6776830.
- ^ "Chagas' Disease". Seattle Biomedical Research Institute. 2008. http://www.sbri.org/diseases/chagas.asp. Retrieved on 2008-09-01.
- ^ Pinto-Dias JC (1992). "Epidemiology of Chagas disease" in ISBT Brazil '92. XXII Congress of the International Society of Blood Transfusion. XX Brazilian Congress of Hematology. Extraordinary Congress of the Brazilian College of Hematology. Wendel S, Brener Z, Camargo ME, Rassi A (eds.) Chagas Disease—American Trypanosomiasis: its impact on transfusion and clinical medicine, São Paulo: Editorial ISBT Brazil. Retrieved on 2008-09-11.
- ^ Teixeira AR, Monteiro PS, Rebelo JM, et al (2001). "Emerging Chagas disease: trophic network and cycle of transmission of Trypanosoma cruzi from palm trees in the Amazon". Emerging Infect Dis 7 (1): 100–12. PMID 11266300. http://www.cdc.gov/ncidod/eid/vol7no1/teixeira.htm.
- ^ a b c d Karsten V, Davis C, Kuhn R (June 1992). "Trypanosoma cruzi in wild raccoons and opossums in North Carolina". J Parasitol 78 (3): 547–9. doi:. PMID 1597808.
- ^ a b Barr SC, Brown CC, Dennis VA, Klei TR (August 1991). "The lesions and prevalence of Trypanosoma cruzi in opossums and armadillos from southern Louisiana". J Parasitol 77 (4): 624–7. doi:. PMID 1907654.
- ^ Yabsley MJ, Noblet GP (January 2002). "Seroprevalence of Trypanosoma cruzi in raccoons from South Carolina and Georgia". J Wildl Dis 38 (1): 75–83. PMID 11838232. http://www.jwildlifedis.org/cgi/pmidlookup?view=long&pmid=11838232.
- ^ Yaeger RG (March 1988). "The prevalence of Trypanosoma cruzi infection in armadillos collected at a site near New Orleans, Louisiana". Am J Trop Med Hyg 38 (2): 323–6. PMID 3128127. http://www.ajtmh.org/cgi/pmidlookup?view=long&pmid=3128127.
- ^ Burkholder JE, Allison TC, Kelly VP (April 1980). "Trypanosoma cruzi (Chagas) (Protozoa: Kinetoplastida) in invertebrate, reservoir, and human hosts of the lower Rio Grande valley of Texas". J Parasitol 66 (2): 305–11. doi:. PMID 6771371.
- ^ Kirchhoff LV (August 1993). "American trypanosomiasis (Chagas' disease)—a tropical disease now in the United States". N Engl J Med 329 (9): 639–44. PMID 8341339. http://content.nejm.org/cgi/pmidlookup?view=short&pmid=8341339&promo=ONFLNS19.
- ^ "Medical Encyclopedia: Chagas disease". National Institutes of Health. http://www.nlm.nih.gov/medlineplus/ency/article/001372.htm. Retrieved on 2008-09-11.
- ^ Steele LS, MacPherson DW, Kim J, Keystone JS, Gushulak BD (January 2007). "The sero-prevalence of antibodies to Trypanosoma cruzi in Latin American refugees and immigrants to Canada". J Immigr Minor Health 9 (1): 43–7. doi:. PMID 17006766.
- ^ a b WHO (2004). The Southern Cone Initiative: an update. Press release. http://www.who.int/tdr/publications/tdrnews/news65/chagas.htm. Retrieved on 2008-08-29.
- ^ Massad E (September 2008). "The elimination of Chagas' disease from Brazil". Epidemiol Infect 136 (9): 1153–64. doi:. PMID 18053273. http://journals.cambridge.org/abstract_S0950268807009879.
- ^ Ramos Jr AN, Carvalho DM (2001). "Os diferentes significados da certificação conferida ao Brasil como estando livre da doença de Chagas" (in Portuguese). Cadernos de Saúde Pública 17 (6): 1403–12. doi:. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-311X2001000600024&lng=en&nrm=iso.
- ^ Pires HH, Lazzari CR, Diotaiuti L, Lorenzo MG (June 2000). "Performance of yeast-baited traps with Triatoma sordida, Triatoma brasiliensis, Triatoma pseudomaculata, and Panstrongylus megistus in laboratory assays". Rev Panam Salud Publica 7 (6): 384–8. doi:. PMID 10949899. http://openurl.ingenta.com/content/nlm?genre=article&issn=1020-4989&volume=7&issue=6&spage=384&aulast=Pires.
- ^ Luz C, Rocha LF, Nery GV, Magalhães BP, Tigano MS (March 2004). "Activity of oil-formulated Beauveria bassiana against Triatoma sordida in peridomestic areas in Central Brazil". Mem Inst Oswaldo Cruz 99 (2): 211–8. doi:. PMID 15250478. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0074-02762004000200017&lng=en&nrm=iso&tlng=en.
- ^ Beard CB, Cordon-Rosales C, Durvasula RV (2002). "Bacterial symbionts of the Triatominae and their potential use in control of Chagas disease transmission". Annu Rev Entomol 47: 123–41. doi:. PMID 11729071. http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.ento.47.091201.145144?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dncbi.nlm.nih.gov.
[edit] Further reading
- "International Symposium to commemorate the 90th anniversary of the discovery of Chagas disease (Rio de Janeiro, April 11–16, 1999)". Memorias do Instituto Oswaldo Cruz 94 (Suppl. I). 1999. http://www.scielo.br/scielo.php?script=sci_issuetoc&pid=0074-027619990007&lng=en&nrm=iso. A Special issue of the Memórias do Instituto Oswaldo Cruz, covering all aspects of Chagas Disease
- Franco-Paredes C (2007). "Chagas disease: an impediment in achieving the Millennium Development Goals in Latin America". BMC International Health and Human Rights 7: 7. doi:. PMID 17725836. http://www.biomedcentral.com/1472-698X/7/7/abstract.
- Miles, Michael W.; Kevin M. Tyler (2003). American trypanosomiasis. Boston: Kluwer Academic Publishers. ISBN 1-4020-7323-2. http://books.google.com/books?id=aSKJbwiBRf8C.
[edit] External links
| Wikimedia Commons has media related to: Chagas disease |
- Chagas disease at the Open Directory Project
- International Symposium on the Centenary of Chagas Disease Discovery - http://www.chagas2009.com.br/
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||







