Hydrocodone
From Wikipedia, the free encyclopedia
 |
|
 |
|
|
Hydrocodone
|
|
| Systematic (IUPAC) name | |
| 4,5a-Epoxy-3-methoxy-17-methylmorphinan-6-one | |
| Identifiers | |
| CAS number | |
| ATC code | R05 |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| Chemical data | |
| Formula | C18H21NO3 |
| Mol. mass | 299.368 |
| SMILES | & |
| Synonyms | dihydrocodeinone |
| Pharmacokinetic data | |
| Bioavailability | High |
| Metabolism | Hepatic |
| Half life | 3.8 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
Category C (USA) |
| Legal status |
Class A(UK) Schedule II in bulk quantities or as stand-alone product; Schedule III when in combination product (USA) |
| Routes | Oral, intravenous, intramuscular, subcutaneous, sublingual, intranasal |
Hydrocodone or dihydrocodeinone is a semi-synthetic opioid derived from two of the naturally occurring opiates codeine and thebaine. Hydrocodone is an orally active narcotic analgesic and antitussive. It is commonly available in tablet, capsule, and syrup form, and is often compounded with other analgesics like paracetamol or ibuprofen. It is marketed, in its varying forms, under a number of trademarks, including Vicodin, Symtan, Anexsia, Dicodid, Hycodan (or generically Hydromet), Hycomine, Hycet, Lorcet, Lortab, Norco, Novahistex, Hydrovo, Duodin, Kolikodol, Orthoxycol, Mercodinone, Synkonin, Norgan, and Hydrokon. Hydrocodone was first synthesized in Germany in 1920[1] and was approved by the FDA on 23 March 1943 for sale in the United States under the brand name Hycodan.[2][3]
The particular niche in which hydrocodone is most commonly used is as an intermediate-strength centrally acting analgesic and strong cough suppressant, especially in those for whom histamine release and attendant itching from codeine is a problem. For the latter indication, at the 5- to 10-mg dose range, hydrocodone is more powerful than most cough suppressants, being roughly equal to its derivative Dihydrocodeinone enol acetate, with the top of the list being hydromorphone (Dilaudid Cough Syrup) and methadone (Methadone linctus, about 33 percent the concentration of the liquid used for opioid physical dependence maintenance or detoxification).
Contents |
[edit] Overview
As a narcotic, hydrocodone relieves pain by binding to opioid receptors in the brain and spinal cord. It can be taken with or without food as desired. When taken with alcohol, it can intensify drowsiness. It may interact with monoamine oxidase inhibitors, as well as other drugs that cause drowsiness. It is in FDA pregnancy category C: Animal reproduction studies have shown an adverse effect on the fetus, and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks. In addition, a newborn of a mother taking the medication may exhibit breathing problems or withdrawal symptoms.
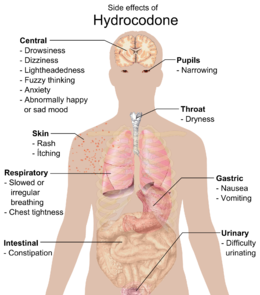
Common side effects include dizziness, lightheadedness, nausea, drowsiness, constipation, vomiting, and euphoria. Vomiting in some patients is so severe that hospitalization is required. Some less common side effects are allergic reaction, blood disorders, changes in mood, mental fogginess, anxiety, lethargy, difficulty urinating, spasm of the ureter, irregular or depressed respiration, and rash.
Studies have shown hydrocodone as stronger than codeine but only one-tenth as potent as morphine at binding to receptors, whereas roughly half as potent as morphine in analgesic properties.[5] However other studies have shown hydrocodone to be anywhere from equally as potent to oxycodone (1.5× the potency of morphine) to only 66.6~% the potency of oxycodone (equipotent to morphine).[6] Hydrocodone can become habit-forming, which leads to physical and psychological addiction, but the potential for addiction varies from individual to individual depending on unique biological differences. Sales and production of this drug have increased significantly in recent years, as have diversion and illicit use. In the U.S., pure hydrocodone and forms containing more than 15 mg per dosage unit are considered Schedule II drugs. Those containing less than or equal to 15 mg per dosage unit in combination with acetaminophen or another non-controlled drug are called hydrocodone compounds and are considered Schedule III drugs. Hydrocodone is typically found in combination with other drugs such as paracetamol, aspirin, ibuprofen and homatropine methylbromide. The purpose of the non-controlled drugs in combination is often twofold: 1) To provide increased analgesia via drug synergy. 2) To limit the intake of hydrocodone by causing unpleasant and often unsafe side effects at higher-than-prescribed doses (See Below). In the UK, it is listed as a Class A drug under the Misuse of Drugs Act 1971. Hydrocodone is not available in pure form in the United States due to a separate regulation, and is always sold with an NSAID, acetaminophen, antihistamine, expectorant, or homatropine. The cough preparation Codiclear DH is the purest US hydrocodone item, containing guaifenesin and small amounts of ethanol as active ingredients. In Germany and elsewhere, hydrocodone is available as single-active-ingredient tablets as Dicodid (by analogy to the original manufacturer's other products Dilaudid and Dinarkon and others) available in 5- and 10-mg strengths.
As with many other opioids, it is quite possible to reduce the amount of hydrocodone needed to stop a certain level of pain by having the patient take the hydrocodone along with one of the medications with analgesic-sparing properties, also known as potentiators. The most common, one of the most effective with hydrocodone, and safest is hydroxyzine. Orphenadrine, nefopam, carisoprodol, and antihistamines also potentiate most opioids. Especially in the case of carisoprodol, it is imperative that the titration and addition of the potentiator be done under strict supervision of a physician.
Hydrocodone also interacts relatively well with most adjuvant and atypical analgesics used for severe and neuropathic pain such as first-generation anti-depressants, anticholinergics, anticonvulsants, centrally acting stimulants, NMDA antagonists, etc. Hydrocodone can usually be successfully used with duloxetine (Cymbalta®) for neuropathic pain, especially that from diabetic neuropathy, provided that the patient has normal relative and absolute levels of Cytochrome P450-related liver enzymes.
Some of the effects of hydrocodone come from the fact that a fraction of it is changed to hydromorphone in the liver, as is the case with all codeine-based analgesics (codeine into morphine, dihydrocodeine into dihydromorphine, nicocodeine into nicomorphine etc.). The percentage can vary based on both other medications taken and inherited metabolic quirks involving the Cytochrome P450 metabolic pathways — some cannot process it at all, whereas a smaller percentage can get even more strength from it than usual. These factors can also cause hydrocodone and related drugs to have a threshold effect, cause significant lengthening or shortening of the duration of effects in the absence of tolerance, and increase or decrease the de facto conversion ratio betwixt hydrocodone and other drugs like morphine, hydromorphone, and synthetics like levorphanol and methadone.
[edit] Overdosing risks
The presence of acetaminophen in hydrocodone-containing products deters many drug users from taking excessive amounts. However, some users will get around this by extracting a portion of the acetaminophen using cold water, taking advantage of the water-soluble element of the drug. It is not uncommon for addicts to have liver problems from consuming excessive amounts of acetaminophen over a long period of time; taking 10,000 to 15,000 milligrams (10 to 15 grams) of acetaminophen in a period of 24 hours typically results in severe hepatotoxicity, and doses in the range of 15,000–20,000 milligrams a day have been reported as fatal.[8] It is this factor that leads many recreational users to use only single-entity opiates such as oxycodone. One of the major problems today with the illicit use of hydrocodone, especially in younger populations, is that users may not be aware that hydrocodone pills contain acetaminophen. Consuming more than 4,000 milligrams of acetaminophen a day can cause liver damage, jaundice, and even liver failure[9] if the drug is being taken in narcotic effect seeking dosages for an extended period of time.
Daily consumption of hydrocodone should not exceed 40 milligrams in patients not tolerant to opiates. However, the 2006 PDR (Physicians Desk Reference) clearly states that Norco 10, containing 10 milligrams of hydrocodone and 325 milligrams of APAP (viz., acetaminophen or paracetamol), can be taken at a dosage of up to twelve tablets per day (120 milligrams of hydrocodone). Such high amounts of hydrocodone are only intended for opiate-tolerant patients, and titration to such levels must be monitored very carefully. This restriction is only limited by the fact that twelve tablets, each containing 325 milligrams of APAP, puts the patient right below the 24-hour FDA maximum of 4,000 mg of APAP. Some specially compounded products are routinely given to chronic pain patients in doses of up to 180 mg of hydrocodone per day. Symptoms of hydrocodone overdosage include respiratory depression, extreme somnolence, coma, stupor, cold and/or clammy skin, sometimes bradycardia, and hypotension. A severe overdose may involve circulatory collapse cardiac arrest and/or death. Mixing hydrocodone with alcohol, cocaine, amphetamines, methylphenidate, benzodiazapines, barbiturates, and a number of other medication can have severe adverse reactions including but not limited to heart failure, heart attack, respiratory distress, pulmonary failure, liver or kidney failure, jaundice, amnesia, seizures, blackouts, and coma. Mixing acetaminophen with other NSAID analgesics like sulindac can cause serious damage to organs.
Hydrocodone in particular, and the -codone family of opioids in general, have been shown to have a liability to cause long term hearing loss over periods of use.[10][11]
[edit] Alcohol
It is not recommended to mix any amounts of hydrocodone and alcohol, as doing so could cause health problems. APAP is metabolized solely by the liver. Therefore the risk of fatal overdose due to hepatotoxicity can occur with significantly lower levels of APAP when mixed with ethanol. Also the mixture can potentially cause serious damage to the liver, kidneys, and stomach wall. Acetaminophen may increase the potential for coma, respiratory problems, and can damage the CNS.[12] Due to the feeling of euphoria it provides, these potentially negative consequences are often ignored by physically and/or psychologically dependent users.
[edit] Hydrocodone compounds
When sold commercially in the United States, hydrocodone is always combined with another medication. Those combined with acetaminophen are known by various trademark names, such as Vicodin and Lortab. Hydrocodone also can be combined with aspirin (e.g., Lortab ASA), ibuprofen (e.g., Vicoprofen), and certain antihistamines (e.g., Chemdal HD).
Combining an opioid such as hydrocodone with another analgesic can increase the effectiveness of the drug without increasing opioid-related side effects (e.g., nausea, constipation, sedation). Another argument for combining hydrocodone with acetaminophen is that it limits the potential for abuse. In tolerant users, hydrocodone can be taken in large doses relatively safely, but acetaminophen is fatally toxic to the liver in large quantities.
[edit] FDA DESI Hydrocodone Cough Preparation Review
Hydrocodone was until recently the active antitussive in more than 200 formulations of cough syrups and tablets sold in the United States. In late 2006, the FDA began forcing the recall of many of these formulations due to reports of deaths in infants and children under the age of six. The legal status of drug formulations originally sold between 1938 and 1962 - before FDA approval was required - was ambiguous. As a result of FDA enforcement action, 88% of the hydrocodone-containing medications have been removed from the market.[citation needed]
At the present time, doctors, pharamacists, and codeine-sensitive or allergic patients or sensitive to the amounts of histamine released by its metabolites must choose among rapidly dwindling supplies of the Hycodan-Codiclear-Hydromet type syrups, Tussionex (an extended-release suspension similar to the European products Codipertussin (codeine hydrochloride) Paracodin suspension (dihydrocodeine hydroiodide), Tusscodin Retard (nicocodeine hydrochloride) and others), and a handful of weak dihydrocodeine syrups. The low sales volume and Schedule II status of Dilaudid Cough Syrup predictably leads to under-utilisation of the drug. There are several conflicting views concerning the US availability of cough preparations containing dionine (aka codethyline and ethylmorphine) — Feco Syrup® and its equivalents were first marketed circa 1895 and still in common use in the 1940s and 1950s, and the main ingredient is treated like codeine in the Controlled Substances Act of 1970.
[edit] See also
[edit] References
- ^ Mannich, C.; Löwenheim, H. (1920). "Ueber zwei neue Reduktionsprodukte des Kodeins". Archiv der Pharmazie 258: 295–316. doi:.
- ^ "Drugs@FDA - Approval History: Hycodan". FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Overview&DrugName=HYCODAN. Retrieved on 2006-01-07.
- ^ "FDA Docket No. 2007N-0353, Drug Products Containing Hydrocodone; Enforcement Action Dates". FDA. http://www.fda.gov/OHRMS/DOCKETS/98fr/E7-19340.htm. Retrieved on 2006-01-07. See section I. B., DESI Review of Hydrocodone Products
- ^ a b MedlinePlus (The American Society of Health-System Pharmacists) - Drug Information: Hydrocodone. Last Revised - 10/01/2008. Retrieved on 02/21/2009.
- ^ Davis, Mellar P. (2005). "Hydrocodone". Opioids for cancer pain. Oxford UK: Oxford University Press. pp. 59–68. ISBN 0-19-852943-0. http://books.google.com/books?id=BK0WduGnx2kC&pg=PA59&lpg=PA59&dq=potency+of+hydrocodone&source=web&ots=yVunxwSuUc&sig=FOj2j1j5PDtGaQr0UJAJ4DyVZXY&hl=en&sa=X&oi=book_result&resnum=2&ct=result.
- ^ Drugs.com - Hydrocodone potency
- ^ a b [http://www.nlm.nih.gov/medlineplus/druginfo/meds/a601006.html MedlinePlus drug information: Hydrocodone. Last revised: Last Revised - 10/01/2008. Retrieved on 15 Mars, 2009
- ^ Rx List
- ^ Acetaminophen (Tylenol) - side effects, drug class, medical uses, and drug interactions by MedicineNet.com
- ^ Friedman RA, House JW, Luxford WM, Gherini S, Mills D (Mar 2000). "Profound hearing loss associated with hydrocodone/acetaminophen abuse". Am J Otol 21 (2): 188–91. doi:. PMID 10733182.
- ^ Ho T, Vrabec JT, Burton AW (May 2007). "Hydrocodone use and sensorineural hearing loss". Pain Physician 10 (3): 467–72. PMID 17525781. http://www.painphysicianjournal.com/linkout_vw.php?issn=1533-3159&vol=10&page=467.
- ^ Draganov, P.; Durrence, H.; Cox, C.; Reuben, A. (Jan 2000). "Alcohol-acetaminophen syndrome" (archived page). Postgraduate Medicine 107 (1). http://web.archive.org/web/20071011203610/postgradmed.com/issues/2000/01_00/draganov.htm.
[edit] External links
|
|||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||




