Speciation
From Wikipedia, the free encyclopedia
| Part of the Biology series on |
| Evolution |
| Introduction |
| Mechanisms and processes |
|
Adaptation |
| Research and history |
|
Evidence |
| Evolutionary biology fields |
|
Cladistics |
| Biology Portal · |
Speciation is the evolutionary process by which new biological species arise. The biologist Orator F. Cook seems to have been the first to coin the term 'speciation' for the splitting of lineages or 'cladogenesis,' as opposed to 'anagenesis' or 'phyletic evolution' occurring within lineages.[1][2] Whether speciation is achieved normally via genetic drift or natural selection is the subject of much ongoing discussion. There are four geographic modes of speciation in nature, based on the extent to which speciating populations are geographically isolated from one another: allopatric, peripatric, parapatric, and sympatric. Speciation may also be induced artificially, through animal husbandry or laboratory experiments. Observed examples of each kind of speciation are provided throughout.[3]
Contents |
[edit] Natural speciation
All forms of natural speciation have taken place over the course of evolution, though it still remains a subject of debate as to the relative importance of each mechanism in driving biodiversity.[4]
A good example of natural speciation is observed repeatedly in the three-spined stickleback, a marine fish which frequently undergoes parapatric speciation into new freshwater colonies at the mouths of rivers. Over typically 10,000 generations, the stickleback may lose their pelvic hind fins, gain tougher bony armor in their scales, and lighten in color as they adapt to life in fresh water.[5]
There is debate as to the rate at which speciation events occur over geologic time. While some evolutionary biologists claim that speciation events have remained relatively constant over time, some palaeontologists such as Niles Eldredge and Stephen Jay Gould have argued that species usually remain unchanged over long stretches of time, and that speciation occurs only over relatively brief intervals, a view known as punctuated equilibrium.
[edit] Allopatric
During allopatric speciation, a population splits into two geographically isolated allopatric populations (for example, by habitat fragmentation due to geographical change such as mountain building or social change such as emigration). The isolated populations then undergo genotypic and/or phenotypic divergence as they (a) become subjected to dissimilar selective pressures or (b) they independently undergo genetic drift. When the populations come back into contact, they have evolved such that they are reproductively isolated and are no longer capable of exchanging genes.
- Observed instances
Island genetics, the tendency of small, isolated genetic pools to produce unusual traits, has been observed in many circumstances, including insular dwarfism and the radical changes among certain famous island chains, like Komodo and Galápagos, the latter having given rise to the modern expression of evolutionary theory, after being observed by Charles Darwin. Perhaps the most famous example of allopatric speciation is Darwin's Galápagos Finches.
[edit] Peripatric
In peripatric speciation, new species are formed in isolated, small peripheral populations which are prevented from exchanging genes with the main population. It is related to the concept of a founder effect, since small populations often undergo bottlenecks. Genetic drift is often proposed to play a significant role in peripatric speciation.
- Observed instances
- Mayr bird fauna
- The Australian bird Petroica multicolor
- Reproductive isolation occurs in populations of Drosophila subject to population bottlenecking
The London Underground mosquito is a variant of the mosquito Culex pipiens which entered in the London Underground in the nineteenth century. Evidence for its speciation include genetic divergence, behavioral differences, and difficulty in mating.[6]
[edit] Parapatric
In parapatric speciation, the zones of two diverging populations are separate but do overlap. There is only partial separation afforded by geography, so individuals of each species may come in contact or cross the barrier from time to time, but reduced fitness of the heterozygote leads to selection for behaviours or mechanisms which prevent breeding between the two species.
Ecologists refer to parapatric and peripatric speciation in terms of ecological niches. A niche must be available in order for a new species to be successful.
- Observed instances
- The three-spined stickleback[5]
- Ring species
- The Larus gulls form a ring species around the North Pole.
- The Ensatina salamanders, which form a ring round the Central Valley in California.
- The Greenish Warbler (Phylloscopus trochiloides), around the Himalayas.
- the grass Anthoxanthum has been known to undergo parapatric speciation in such cases as mine contamination of an area.
[edit] Sympatric
| This date:March, 2008 needs additional citations for verification. Please help improve this article by adding reliable references (ideally, using inline citations). Unsourced material may be challenged and removed. (March 2008) |
In sympatric speciation, species diverge while inhabiting the same place. Often cited examples of sympatric speciation are found in insects which become dependent on different host plants in the same area. However, the existence of sympatric speciation as a mechanism of speciation is still hotly contested. People have argued that the evidences of sympatric speciation are in fact examples of micro-allopatric, or heteropatric speciation. The most widely accepted example of sympatric speciation is that of the cichlids of Lake Nabugabo in East Africa, which is thought to be due to sexual selection. Sympatric speciation refers to the formation of two or more descendant species from a single ancestral species all occupying the same geographic location.
Until recently, there has a been a dearth of hard evidence that supports this form of speciation, with a general feeling that interbreeding would soon eliminate any genetic differences that might appear. But there has been at least one recent study that suggests that sympatric speciation has occurred in Tennessee cave salamanders.[7]
The three-spined sticklebacks, freshwater fishes, that have been studied by Dolph Schluter (who received his Ph.D. for his work on Darwin's finches with Peter Grant) and his current colleagues in British Columbia, provide an intriguing example that is best explained by sympatric speciation. They have found:
- Two different species of three-spined sticklebacks in each of five different lakes.
- a large benthic species with a large mouth that feeds on large prey in the littoral zone
- a smaller limnetic species — with a smaller mouth — that feeds on the small plankton in open water.
- DNA analysis indicates that each lake was colonized independently, presumably by a marine ancestor, after the last ice age.
- DNA analysis also shows that the two species in each lake are more closely related to each other than they are to any of the species in the other lakes.
- Nevertheless, the two species in each lake are reproductively isolated; neither mates with the other.
- However, aquarium tests showed that
- the benthic species from one lake will spawn with the benthic species from the other lakes and
- likewise the limnetic species from the different lakes will spawn with each other.
- These benthic and limnetic species even display their mating preferences when presented with sticklebacks from Japanese lakes; that is, a Canadian benthic prefers a Japanese benthic over its close limnetic cousin from its own lake.
- Their conclusion: in each lake, what began as a single population faced such competition for limited resources that
- disruptive selection — competition favoring fishes at either extreme of body size and mouth size over those nearer the mean — coupled with
- assortative mating — each size preferred mates like it - favored a divergence into two subpopulations exploiting different food in different parts of the lake.
- The fact that this pattern of speciation occurred the same way on three separate occasions suggests strongly that ecological factors in a sympatric population can cause speciation.
Sympatric speciation driven by ecological factors may also account for the extraordinary diversity of crustaceans living in the depths of Siberia's Lake Baikal.
[edit] Speciation via polyploidization

Polyploidy is a mechanism often attributed to causing some speciation events in sympatry. Not all polyploids are reproductively isolated from their parental plants, so an increase in chromosome number may not result in the complete cessation of gene flow between the incipient polyploids and their parental diploids (see also hybrid speciation).
Polyploidy is observed in many species of both plants and animals. In fact, it has been proposed that all of the existing plants and most of the animals are polyploids or have undergone an event of polyploidization in their evolutionary history. However, reproduction is often by parthenogenesis since polyploid animals are often sterile. Rare instances of polyploid mammals are known, but most often result in prenatal death.
[edit] Speciation via hybrid formation
See Hybrid speciation section under the Genetics heading below.
[edit] Reinforcement (Wallace effect)
Reinforcement is the process by which natural selection increases reproductive isolation.[8] It may occur after two populations of the same species are separated and then come back into contact. If their reproductive isolation was complete, then they will have already developed into two separate incompatible species. If their reproductive isolation is incomplete, then further mating between the populations will produce hybrids, which may or may not be fertile. If the hybrids are infertile, or fertile but less fit than their ancestors, then there will be no further reproductive isolation and speciation has essentially occurred (e.g., as in horses and donkeys.) The reasoning behind this is that if the parents of the hybrid offspring each have naturally selected traits for their own certain environments, the hybrid offspring will bear traits from both, therefore would not fit either ecological niche as well as the parents did. The low fitness of the hybrids would cause selection to favor assortative mating, which would control hybridization. This is sometimes called the Wallace effect after the evolutionary biologist Alfred Russel Wallace who suggested in the late 19th century that it might be an important factor in speciation.[9] If the hybrid offspring are more fit than their ancestors, then the populations will merge back into the same species within the area they are in contact.
Reinforcement is required for both parapatric and sympatric speciation. Without reinforcement, the geographic area of contact between different forms of the same species, called their "hybrid zone," will not develop into a boundary between the different species. Hybrid zones are regions where diverged populations meet and interbreed. Hybrid offspring are very common in these regions, which are usually created by diverged species coming into secondary contact. Without reinforcement the two species would have uncontrollable inbreeding. Reinforcement may be induced in artificial selection experiments as described below.
[edit] Artificial speciation
New species have been created by domesticated animal husbandry, but the initial dates and methods of the initiation of such species are not clear. For example, domestic sheep were created by hybridisation, and no longer produce viable offspring with Ovis orientalis, one species from which they are descended.[10] Domestic cattle, on the other hand, can be considered the same species as several varieties of wild ox, gaur, yak, etc., as they readily produce fertile offspring with them.[11]
The best-documented creations of new species in the laboratory were performed in the late 1980s. William Rice and G.W. Salt bred fruit flies, Drosophila melanogaster, using a maze with three different choices of habitat such as light/dark and wet/dry. Each generation was placed into the maze, and the groups of flies which came out of two of the eight exits were set apart to breed with each other in their respective groups. After thirty-five generations, the two groups and their offspring were isolated reproductively because of their strong habitat preferences: they mated only within the areas they preferred, and so did not mate with flies that preferred the other areas.[12] The history of such attempts is described in Rice and Hostert (1993).[13]
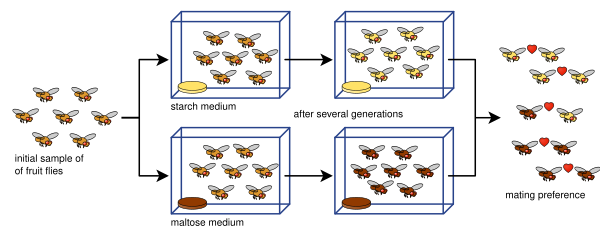
Diane Dodd was also able to show how mating preferences can develop from reproductive isolation in Drosophila pseudoobscura fruit flies after only eight generations using different food types, starch and maltose.[14]
Dodd's experiment has been easy for many others to replicate, including with other kinds of fruit flies and foods.[15]
[edit] Genetics
Few speciation genes have been found. They usually involve the reinforcement process of late stages of speciation. In 2008 a speciation gene causing reproductive isolation was reported.[16] It causes hybrid sterility between related subspecies.
[edit] Hybrid speciation
Hybridization between two different species sometimes leads to a distinct phenotype. This phenotype can also be fitter than the parental lineage and as such natural selection may then favor these individuals. Eventually, if reproductive isolation is achieved, it may lead to a separate species. However, reproductive isolation between hybrids and their parents is particularly difficult to achieve and thus hybrid speciation is considered an extremely rare event. The Mariana Mallard arose from hybrid speciation.
Hybridization without change in chromosome number is called homoploid hybrid speciation. It is considered very rare but has been shown in Heliconius butterflies [17] and sunflowers. Polyploid speciation, which involves changes in chromosome number, is a more common phenomenon, especially in plant species.
[edit] Gene transposition as a cause
Theodosius Dobzhansky, who studied fruit flies in the early days of genetic research in 1930s, speculated that parts of chromosomes that switch from one location to another might cause a species to split into two different species. He mapped out how it might be possible for sections of chromosomes to relocate themselves in a genome. Those mobile sections can cause sterility in inter-species hybrids, which can act as a speciation pressure. In theory, his idea was sound, but scientists long debated whether it actually happened in nature. Eventually a competing theory involving the gradual accumulation of mutations was shown to occur in nature so often that geneticists largely dismissed the moving gene hypothesis.[18]
However, 2006 research shows that jumping of a gene from one chromosome to another can contribute to the birth of new species.[19] This validates the reproductive isolation mechanism, a key component of speciation.[20]
[edit] Interspersed repeats
Interspersed repetitive DNA sequences function as isolating mechanisms. These repeats protect newly evolving gene sequences from being overwritten by gene conversion, due to the creation of non-homologies between otherwise homologous DNA sequences. The non-homologies create barriers to gene conversion. This barrier allows nascent novel genes to evolve without being overwritten by the progenitors of these genes. This uncoupling allows the evolution of new genes, both within gene families and also allelic forms of a gene. The importance is that this allows the splitting of a gene pool without requiring physical isolation of the organisms harboring those gene sequences.
[edit] Human speciation
Humans have genetic similarities with chimpanzees and gorillas, suggesting common ancestors. Analysis of genetic drift and recombination using a Markov model suggests humans and chimpanzees speciated apart 4.1 million years ago.[21]
[edit] See also
[edit] References
- ^ Cook, O. F. 1906. Factors of species-formation. Science 23:506-507.
- ^ Cook, O. F. 1908. Evolution without isolation. American Naturalist 42:727-731.
- ^ Observed Instances of Speciation by Joseph Boxhorn. Retrieved 28 October 2006.
- ^ J.M. Baker (2005). "Adaptive speciation: The role of natural selection in mechanisms of geographic and non-geographic speciation". Studies in History and Philosophy of Biological and Biomedical Sciences 36: 303–326. doi:. available online
- ^ a b Kingsley, D.M. (January 2009) "From Atoms to Traits," Scientific American, p. 57
- ^ Katharine Byrne and Richard A Nichols (1999) "Culex pipiens in London Underground tunnels: differentiation between surface and subterranean populations"
- ^ MATTHEW L. NIEMILLER, BENJAMIN M. FITZPATRICK, BRIAN T. MILLER (2008). "Recent divergence with gene flow in Tennessee cave salamanders (Plethodontidae: Gyrinophilus) inferred from gene genealogies". Molecular Ecology 17 (9): 2258–2275. available online
- ^ Ridley, M. (2003) "Speciation - What is the role of reinforcement in speciation?" adapted from Evolution 3rd edition (Boston: Blackwell Science) tutorial online
- ^ Ollerton, J. "Flowering time and the Wallace Effect" (PDF). Heredity, August 2005. http://oldweb.northampton.ac.uk/aps/env/lbrg/journals/papers/OllertonHeredityCommentary2005.pdf. Retrieved on 2007-05-22.
- ^ Hiendleder S., et al. (2002) "Molecular analysis of wild and domestic sheep questions current nomenclature and provides evidence for domestication from two different subspecies" Proceedings of the Royal Society B: Biological Sciences 269:893-904
- ^ Nowak, R. (1999) Walker's Mammals of the World 6th ed. (Baltimore: Johns Hopkins University Press)
- ^ Rice, W.R. and G.W. Salt (1988). "Speciation via disruptive selection on habitat preference: experimental evidence". The American Naturalist 131: 911–917. doi:.
- ^ W.R. Rice and E.E. Hostert (1993). "Laboratory experiments on speciation: What have we learned in forty years?". Evolution 47: 1637–1653. doi:.
- ^ Dodd, D.M.B. (1989) "Reproductive isolation as a consequence of adaptive divergence in Drosophila pseudoobscura." Evolution 43:1308–1311.
- ^ Kirkpatrick, M. and V. Ravigné (2002) "Speciation by Natural and Sexual Selection: Models and Experiments" The American Naturalist 159:S22–S35 DOI
- ^ http://www.sciencemag.org/cgi/content/short/323/5912/376
- ^ Mavarez, J.; Salazar, C.A., Bermingham, E., Salcedo, C., Jiggins, C.D. , Linares, M. (2006). "Speciation by hybridization in Heliconius butterflies". Nature 441: 868. doi:.
- ^ University of Rochester Press Releases
- ^ Masly, John P., Corbin D. Jones, Mohamed A. F. Noor, John Locke, and H. Allen Orr (September 2006). "Gene Transposition as a Cause of Hybrid Sterility in Drosophila". Science 313 (5792): 1448–1450. doi:. PMID 16960009. http://www.sciencemag.org/cgi/content/short/313/5792/1448. Retrieved on 2007-03-18.
- ^ Minkel, J.R. (September 8, 2006) "Wandering Fly Gene Supports New Model of Speciation" Science News
- ^ Hobolth A, Christensen OF, Mailund T, Schierup MH (2007) "Genomic Relationships and Speciation Times of Human, Chimpanzee, and Gorilla Inferred from a Coalescent Hidden Markov Model." PLoS Genet 3(2): e7 (doi:10.1371/journal.pgen.0030007)
[edit] Further reading
- Coyne, J. A. & Orr, H. A. (2004). Speciation. Sunderlands, Massachusetts: Sinauer Associates, Inc. ISBN 0-87893-089-2.
- Grant, V. (1981). Plant Speciation (2nd Edit. ed.). New York: Columbia University Press. ISBN 0-231-05113-1.
- Mayr, E. (1963). Animal Species and Evolution. Harvard University Press. ISBN 0-674-03750-2
- White, M. J. D. (1978). Modes of Speciation. San Francisco, California: W. H. Freeman and Company. ISBN 0-716-70284-3.
- Dedicated issue of Philosophical Transactions B on Speciation in microorganisms is freely available.
[edit] External links
- Observed Instances of Speciation from the Talk.Origins Frequently Asked Questions
- Speciation, and
- Evidence for Speciation from Understanding Evolution by the University of California Museum of Paleontology
- Speciation from John Hawks' Anthropology Weblog - paleoanthropology, genetics, and evolution
- Speciation in the context of evolution
|
||||||||||||||
|
|||||||||||||||||||||||||||||