Lactose intolerance
From Wikipedia, the free encyclopedia
| Lactose intolerance Classification and external resources |
|
| Lactose (disaccharide of β-D-galactose & β-D-glucose) is normally split by lactase. | |
| ICD-10 | E73. |
| ICD-9 | 271.3 |
| OMIM | 223100 150220 |
| DiseasesDB | 7238 |
| MedlinePlus | 000276 |
| eMedicine | med/3429 ped/1270 |
| MeSH | D007787 |
Lactose intolerance is the inability to metabolize lactose, a sugar found in milk and other dairy products, because the required enzyme lactase is absent in the intestinal system or its availability is lowered. It is estimated that 75% of adults worldwide show some decrease in lactase activity during adulthood.[1] The frequency of decreased lactase activity ranges from nearly 5% in northern Europe, up to 71% for Southern Europe, to more than 90% in some African and Asian countries.[2]
Contents |
[edit] Overview
Disaccharides cannot be absorbed through the wall of the small intestine into the bloodstream, so in the absence of lactase, lactose present in ingested dairy products remains uncleaved and passes intact into the colon. The operons of enteric bacteria quickly switch over to lactose metabolism, and the resultant in vivo fermentation produces copious amounts of gas (a mixture of hydrogen, carbon dioxide, and methane). This, in turn, may cause a range of abdominal symptoms, including stomach cramps, bloating, and flatulence. In addition, as with other unabsorbed sugars (such as sorbitol, mannitol, and xylitol), the presence of lactose and its fermentation products raises the osmotic pressure of the colon contents.
[edit] Classification
There are three major types of lactose intolerance:[3]
- Primary lactose intolerance. Environmentally induced when weaning a child in non-dairy consuming societies.[4] This is found in many Asian and African cultures, where industrialized and commercial dairy products are uncommon.
- Secondary lactose intolerance. Environmentally induced, resulting from certain gastrointestinal diseases, including exposure to intestinal parasites such as giardia.[5][6] In such cases the production of lactase may be permanently disrupted.[5][6][7] A very common cause of temporary lactose intolerance is gastroenteritis, particularly when the gastroenteritis is caused by rotavirus. Another form of temporary lactose intolerance is lactose overload in infants.[8]
- Congenital lactase deficiency. A genetic disorder which prevents enzymatic production of lactase. Present at birth, and diagnosed in early infancy.
[edit] Lactase biology
The normal mammalian condition is for the young of a species to experience reduced lactase production at the end of the weaning period (a species-specific length of time). In non dairy consuming societies, lactase production usually drops about 90% during the first four years of life, although the exact drop over time varies widely.[9].
However, certain human populations have a mutation on chromosome 2 which eliminates the shutdown in lactase production, making it possible for members of these populations to continue consumption of fresh milk and other dairy products throughout their lives without difficulty. This appears to be an evolutionarily recent adaptation to dairy consumption, and has occurred independently in both northern Europe and east Africa in populations with a historically pastoral lifestyle.[10] Lactase persistence, allowing lactose digestion to continue into adulthood, is a dominant allele, making lactose intolerance a recessive genetic trait.
Some cultures, such as that of Japan, where dairy consumption has been on the increase, demonstrate a lower prevalence of lactose intolerance in spite of a genetic predisposition[11].
Pathological lactose intolerance can be caused by Coeliac disease, which damages the villi in the small intestine that produce lactase. This lactose intolerance is temporary. Lactose intolerance associated with coeliac disease ceases after the patient has been on a gluten-free diet long enough for the villi to recover[citation needed].
Certain people who report problems with consuming lactose are not actually lactose intolerant. In a study of 323 Sicilian adults, Carroccio et al. (1998) found only 4% were both lactose intolerant and lactose maldigesters, while 32.2% were lactose maldigesters but did not test as lactose intolerant. However, Burgio et al. (1984) found that 72% of 100 Sicilians were lactose intolerant in their study and 106 of 208 northern Italians (i.e., 51%) were lactose intolerant.
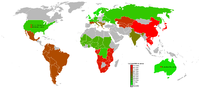
[edit] Lactose intolerance by group
| Human groups | Individuals Examined | Percent Intolerant | Allele frequency |
| Dutch | N/A | 001%[12] | N/A |
| Swedes | N/A | 002%[13] | 0.14 |
| Europeans in Australia | 160 | 004%[13] | 0.20 |
| Northern Europeans and Scandinavians | N/A | 005%[4][14] | N/A |
| Danes | N/A | 005%[15] | N/A |
| Basques | 85 | 008.3%[16] | N/A |
| British | N/A | 005–015%[17] | 0.184-0.302[18] |
| Swiss | N/A | 010%[13] | 0.316 |
| European Americans | 245 | 012%[13] | 0.346 |
| Tuareg | N/A | 013%[17] | N/A |
| Germans | N/A | 015%[17] | N/A |
| Austrians | N/A | 015–020%[17] | N/A |
| Eastern Slavs (Russians, Belarusians, Ukrainians) | N/A | 015%[19] | N/A |
| Northern French | N/A | 017%[17] | N/A |
| Finns | 134 | 018%[13] | 0.424 |
| Central Italians | 65 | 019%[20] | N/A |
| Indians | N/A | 020%[4][14] | N/A |
| African Tutsi | N/A | 020%[13] | 0.447 |
| African Fulani | N/A | 023%[13] | 0.48 |
| Bedouins | N/A | 025%[17] | N/A |
| Portuguese adults | 102 | 035%[21] | N/A |
| African American Children | N/A | 045%[4] | N/A |
| Southern Italians | 51 | 041%[20] | N/A |
| Saami (in Russia and Finland) | N/A | 025–060%[22] | N/A |
| Northern Italians | 89 | 052%[20] | N/A |
| North American Hispanics | N/A | 53%[17] | N/A |
| Balkans | N/A | 055%[17] | N/A |
| Mexican American Males | N/A | 055%[4][14] | N/A |
| Cretans | N/A | 056%[4] | N/A |
| African Maasai | 21 | 062%[23] | N/A |
| Southern French | N/A | 065%[17] | N/A |
| Greek Cypriots | N/A | 066%[4][14] | N/A |
| North American Jews | N/A | 068.8%[4][14] | N/A |
| Sicilians | 100 | 071%[24][25] | N/A |
| South Americans | N/A | 065–075%[17] | N/A |
| Rural Mexicans | N/A | 073.8%[4][14] | N/A |
| African Americans | 20 | 075%[13] | 0.87 |
| Kazakhs from northwest Xinjiang | 195 | 076.4% [26] | |
| Lebanese | 75 | 078%[27] | N/A |
| Central Asians | N/A | 080%[17] | N/A |
| Alaskan Eskimo | N/A | 080%[4][14] | N/A |
| Australian Aborigines | 44 | 085%[13] | 0.922 |
| Inner Mongolians | 198 | 087.9%[26] | |
| African Bantu | 59 | 089%[13] | 0.943 |
| Asian Americans | N/A | 090%[4][14] | N/A |
| Northeastern Han Chinese | 248 | 092.3%[26] | |
| Chinese | 71 | 095%[13] | 0.964 |
| Southeast Asians | N/A | 098%[4][14] | N/A |
| Thais | 134 | 098%[13] | 0.99 |
| Native Americans | 24 | 100%[13] | 1.00 |
The statistical significance varies greatly depending on number of people sampled.
Lactose intolerance levels also increase with age. At ages 2 - 3 yrs., 6 yrs., and 9 - 10 yrs., the amount of lactose intolerance is, respectively:
- 6% to 15% in white Americans and northern Europeans
- 18%, 30%, and 47% in Mexican Americans
- 25%, 45%, and 60% in black South Africans
- approximately 10%, 20%, and 25% in Chinese and Japanese
- 30–55%, 90%, and >90% in Mestizos of Peru[28][29]
Chinese and Japanese populations typically lose between 20 and 30 percent of their ability to digest lactose within three to four years of weaning. Some studies have found that most Japanese can consume 200 ml (8 fl oz) of milk without severe symptoms (Swagerty et al., 2002).[11]
Ashkenazi Jews can keep 20 - 30 percent of their ability to digest lactose for many years.[12][28][30] Of the 10% of the Northern European population that develops lactose intolerance, the development of lactose intolerance is a gradual process spread out over as many as 20 years.[31]
[edit] Diagnosis
To assess lactose intolerance, the intestinal function is challenged by ingesting more dairy than can be readily digested. Clinical symptoms typically appear within 30 minutes but may take up to 1–2 hours depending on other foods and activities.[32] Substantial variability of the clinical response (symptoms of nausea, cramping, bloating, diarrhea, and flatulence) are to be expected as the extent and severity of lactose intolerance varies between individuals.
When considering the need for confirmation, it is important to distinguish lactose intolerance from milk allergy, which is an abnormal immune response (usually) to milk proteins. Since lactose intolerance is the normal state for most adults on a worldwide scale, and not considered a disease condition, a medical diagnosis is not normally required. However, if confirmation is necessary, three tests are available:
[edit] Hydrogen breath test
In a hydrogen breath test, after an overnight fast, 50 grams of lactose (in a solution with water) is swallowed. If the lactose cannot be digested, enteric bacteria metabolize it and produce hydrogen. This, along with methane, can be detected in the patient's breath by a clinical gas chromatograph or a compact solid state detector. The test takes about 2 to 3 hours. A medical condition with similar symptoms is fructose malabsorption.
In conjunction, measuring the blood glucose level every 10 – 15 minutes after ingestion will show a "flat curve" in individuals with lactose malabsorption, while the lactase persistent will have a significant "top", with an elevation of typically 50 to 100% within 1 – 2 hours. However, given the need for frequent blood drawns, this approach has been largely supplanted by breath testing.
[edit] Stool acidity
Can be used to diagnose lactose intolerance in small infants, for whom other forms of testing are risky or impractical.[33]
[edit] Intestinal biopsy
An intestinal biopsy can confirm lactose intolerance following discovery of elevated hydrogen in the hydrogen breath test.[34] However, given the invasive nature of this test, and the need for a highly specialized laboratory to measure lactase enzymes or mRNA in the biopsy tissue, this approach is used almost exclusively in clinical research.
[edit] History of diagnosis
The ancient Greek physician Hippocrates (460-370 B.C.) first noted gastrointestinal upset and skin problems in some who consumed milk;[35] patients experiencing the former symptom may likely have been suffering from lactose intolerance. However, it was only in the last few decades that the syndrome was more widely described by modern medical science.
The condition was first recognized in the 1950s and 1960s when various organizations like the United Nations began to engage in systematic famine-relief efforts in countries outside Europe for the first time. Holzel et al. (1959) and Durand (1959) produced two of the earliest studies of lactose intolerance. As anecdotes of embarrassing dairy-induced discomfort increased, the First World donor countries could no longer ascribe the reports to spoilage in transit or inappropriate food preparation by the Third World recipients.
Because the first nations to industrialize and develop modern scientific medicine were dominated by people of European descent, adult dairy consumption was long taken for granted. Westerners for some time did not recognize that the majority of the human ethno-genetic groups could not consume dairy products during adulthood. Although there had been regular contact between Europeans and non-Europeans throughout history, the notion that large-scale medical studies should be representative of the ethnic diversity of the human populations (as well as all genders and ages) did not become well-established until after the American Civil Rights Movement.[citation needed]
Since then, the relationship between lactase and lactose has been thoroughly investigated in food science due to the growing market for dairy products among non-Europeans.
Originally it was hypothesised that gut bacteria such as E. coli produced the lactase enzyme needed to cleave lactose into its constituent monosaccharides and thus become metabolisable and digestible by humans. Some form of human-bacteria symbiosis was proposed as a means of producing lactase in the human digestive tract. Genetics and protein analysis techniques by the early 1970s revealed this to be untrue; humans produce their own lactase enzyme natively in intestine cells.[citation needed]
According to Heyman (2006), approximately 70% of the global population cannot tolerate lactose in adulthood. Thus, some argue that the terminology should be reversed — lactose intolerance should be seen as the norm, and the minority groups should be labeled as having lactase persistence. A counter argument to this is that the cultures that don't generally consume unmodified milk products have little need to discuss their intolerance to it, leaving the cultures for which lactose intolerance is a significant dietary issue to define its terminology.
[edit] History of genetic prevalence
Lactose intolerance has been studied as an aid in understanding ancient diets and population movement in prehistoric societies. Milking an animal vastly increases the calories that may be extracted from the animal as compared to the consumption of its meat alone. It is not surprising then, that consuming milk products became an important part of the agricultural way of life in the Neolithic. It is believed that most of the milk was used to make mature cheeses which are mostly lactose free.[citation needed]
Roman authors recorded that the people of northern Europe, particularly Britain and Germany drank unprocessed milk (as opposed to the Romans who made cheese).[citation needed] This corresponds very closely with modern European distributions of lactose intolerance, where the people of Britain, Germany and Scandinavia have a good tolerance, and those of southern Europe, especially Italy, have a poorer tolerance.[36]
In east Asia, historical sources also attest that the Chinese did not consume milk, whereas the nomads that lived on the borders did. Again, this reflects modern distributions of intolerance. China is particularly notable as a place of poor tolerance, whereas in Mongolia and the Asian steppes horse milk is drunk regularly. This tolerance is thought to be advantageous as the nomads do not settle down long enough to process mature cheese. Given that their prime source of income is generated through horses, to ignore their milk as a source of calories would be greatly detrimental. The nomads also make an alcoholic beverage, called Kumis, from horse milk, although the fermentation process reduces the amount of lactose present.
The African Fulani have a nomadic origin and their culture once completely revolved around cow, goat, and sheep herding. Dairy products were once a large source of nutrition for them. As might be expected if lactase persistence evolved in response to dairy product consumption, they are particularly tolerant to lactose (about 77% of the population). Many Fulani live in Guinea-Conakry, Burkina Faso, Mali, Nigeria, Niger, Cameroon, and Chad.
There is some debate on exactly where and when genetic mutation(s) occurred. Some argue for separate mutation events in Sweden (which has one of the lowest levels of lactose intolerance in the world) and the Arabian Peninsula around 4000 BC. However, others argue for a single mutation event in the Middle East at about 4500 BC which then subsequently radiated. Some sources suggest a third and more recent mutation in the East African Tutsi. Whatever the precise origin in time and place, most modern Northern Europeans and people of European ancestry show the effects of this mutation (that is, they are able to safely consume milk products all their lives) while most modern East Asians, sub-Saharan Africans and native peoples of the Americas and Pacific Islands do not (making them lactose intolerant as adults).[37] The Maasai ability to consume dairy without exhibiting symptoms may be due to a different genetic mutation.[38] Or it may be due to the fact that they curdle their milk before they consume it, removing the lactose.
A thorough scientific overview of genetic polymorphisms of intestinal lactase activity in adult hypolactasia, is in chapter 76 of OMMBID.[39] A noncoding variation in the MCM6 gene has been strongly associated with adult type hypolactasia.[4]
[edit] Managing lactose intolerance
For persons living in societies where the diet contains relatively little dairy, lactose intolerance is not considered a condition that requires treatment. However, those living among societies that are largely lactose-tolerant may find lactose intolerance troublesome. Although there are still no methodologies to reinstate lactase production, some individuals have reported their intolerance to vary over time (depending on health status and pregnancy[40]). Lactose intolerance is not usually an all-or-nothing condition: the reduction in lactase production, and hence, the amount of lactose that can be tolerated varies from person to person. Since lactose intolerance poses no further threat to a person's health, managing the condition consists of minimizing the occurrence and severity of symptoms. Berdanier and Hargrove recognise 4 general principles: 1) avoidance of dietary lactose; 2) substitution to maintain nutrient intake; 3) regulation of calcium intake; 4) use of enzyme substitute.
[edit] Avoiding lactose-containing products
Since each individual's tolerance to lactose varies, according to the US National Institute of Health, "Dietary control of lactose intolerance depends on people learning through trial and error how much lactose they can handle."[41] Label reading is essential as commercial terminology varies according to language and region.[34]
Lactose is present in 2 large food categories: Conventional dairy products, and as a food additive (in dairy and non dairy products).
[edit] Dairy products
Lactose is a water-soluble molecule. Therefore fat percentage and the curdling process have an impact on which foods may be tolerated. In the curdling process lactose is found in the water portion along with whey and casein, but is not found in the fat portion. Dairy products which are "fat reduced" or "fat free" generally have a slightly higher lactose percentage. Additionally, low fat dairy foods also often have various dairy derivatives such as milk solids added to them to enhance sweetness, increasing the lactose content.
Milk. Human milk has the highest lactose percentage at around 9%. Unprocessed cow milk has 4.7% lactose. Unprocessed milk from other bovids contains similar lactose percentages (goat milk 4.1%,[42] buffalo 4.86%,[43] yak 4.93%,[44] sheep milk 4.6%)
Butter. The butter-making process separates the majority of milk's water components from the fat components. Lactose, being a water soluble molecule, will be present in small quantities in the butter unless it is also fermented to produce cultured butter.
Yogurt and kefir. People can be more tolerant of traditionally made yogurt than milk because it contains lactase enzyme produced by the bacterial cultures used to make the yogurt. However, many commercial brands contain milk solids, increasing the lactose content.
Cheeses. Traditionally made hard cheese (such as Swiss cheese) and soft ripened cheeses may create less reaction than the equivalent amount of milk because of the processes involved. Fermentation and higher fat content contribute to lesser amounts of lactose. Traditionally made Swiss or Cheddar might contain 10% of the lactose found in whole milk. In addition, the traditional aging methods of cheese (over 2 years) reduces their lactose content to practically nothing.[1] Commercial cheese brands, however, are generally manufactured by modern processes that do not have the same lactose reducing properties, and as no regulations mandate what qualifies as an "aged" cheese, this description does not provide any indication of whether the process used significantly reduced lactose.
Sour cream and ice cream, like yogurt, if made the traditional way, may be tolerable, but most modern brands add milk solids.[45] Consult labels.[46]
Examples of lactose levels in foods. As scientific consensus has not been reached concerning lactose percentage analysis methods [47] (non-hydrated form or the mono-hydrated form), and considering that dairy content varies greatly according to labeling practices, geography and manufacturing processes, lactose numbers may not be very reliable. The following are examples of lactose levels in foods which commonly set off symptoms.[41] These quantities are to be treated as guidelines only.
-
Dairy product Lactose Content Yogurt, plain, low-fat, 240 mL 5 g Milk, reduced fat, 240 mL 11 g Swiss cheese, 28 g 1 g Ice cream, 120 mL 6 g Cottage cheese, 120 mL 2–3 g
[edit] Lactose in non-dairy products
Lactose (also present when labels state lactoserum, whey, milk solids, modified milk ingredients, etc) is a commercial food additive used for its texture, flavor and adhesive qualities, and is found in foods such as processed meats[48] (sausages/hot dogs, sliced meats, Pâtés), gravy stock powder, margarines[49] sliced breads,[50][51] breakfast cereals, potato chips,[52] dried fruit, processed foods, medications, preprepared meals, meal replacement (powders and bars), protein supplements (powders and bars).
Kosher products labeled pareve are free of milk. However, if a "D" (for "Dairy) is present next to the circled "K", "U", or other hechsher, the food likely contains milk solids[48] (although it may also simply indicate that the product was produced on equipment shared with other products containing milk derivatives).
[edit] Alternative products
Plant based milks and derivatives are the only[citation needed] ones to be 100% lactose free: soy milk, rice milk, almond milk, hazelnut milk, oat milk, hemp milk, peanut milk, horchata.
The dairy industry has created low-lactose or lactose-free products to replace regular dairy. Lactose-free milk can be produced by passing milk over lactase enzyme bound to an inert carrier: once the molecule is cleaved, there are no lactose ill-effects. A form is available with reduced amounts of lactose (typically 30% of normal), and alternatively with nearly 0%. Finland, where approximately 17% of the Finnish-speaking population has hypolactasia,[53] has had "HYLA" (acronym for hydrolysed lactose) products available for many years. These low-lactose level cow's milk products, ranging from ice cream to cheese, use a Valio patented chromatographic separation method to remove lactose. The ultra-pasteurization process, combined with aseptic packaging, ensures a long shelf-life. Recently, the range of low-lactose products available in Finland has been augmented with milk and other dairy products (such as ice cream, butter, and buttermilk) that contain no lactose at all. The remaining about 20% of lactose in HYLA products is taken care of enzymatically. These typically cost slightly more than equivalent products containing lactose. Valio also markets these products in Sweden and in Estonia.
Alternatively, a bacterium such as L. acidophilus may be added, which affects the lactose in milk the same way it affects the lactose in yogurt (see above).
[edit] Lactase supplementation
When lactose avoidance is not possible, or on occasions when a person chooses to consume such items, then enzymatic lactase supplements may be used.[54][55]
Lactase enzymes similar to those produced in the small intestines of humans are produced industrially by fungi of the genus aspergillus. The enzyme, β-galactosidase, is available in tablet form in a variety of doses, in many countries without a prescription. It functions well only in high-acid environments, such as that found in the human gut due to the addition of gastric juices from the stomach. Unfortunately, too much acid can denature it,[56] and it therefore should not be taken on an empty stomach. Also, the enzyme is ineffective if it does not reach the small intestine by the time the problematic food does. Lactose-sensitive individuals should experiment with both timing and dosage to fit their particular need. But supplements such as these may not be able to provide the accurate amount of lactase needed[citation needed] to adequately digest the lactose contained in dairy products, which may lead to symptoms similar to the existing lactose intolerance.
While essentially the same process as normal intestinal lactose digestion, direct treatment of milk employs a different variety of industrially produced lactase. This enzyme, produced by yeast from the genus kluyveromyces, takes much longer to act, must be thoroughly mixed throughout the product, and is destroyed by even mildly acidic environments. It therefore has been much less popular as a consumer product[citation needed] (sold, where available, as a liquid) than the aspergillus-produced tablets, despite its predictable effectiveness. Its main use is in producing the lactose-free or lactose-reduced dairy products sold in supermarkets.
Enzymatic lactase supplementation may have an advantage over avoiding dairy products, in that alternative provision does not need to be made to provide sufficient calcium intake, especially in children.[57]
[edit] Rehabituation to dairy products
For healthy individuals with secondary lactose intolerance, it may be possible to train bacteria in the large intestine to break down lactose more effectively[2] by consuming small quantities of dairy products several times a day over a couple of weeks. Reintroducing dairy in this way to people who have an underlying or chronic illness, however, is not recommended, as certain illnesses damage the intestinal tract in a way which prevents the lactase enzyme from being expressed.
Some studies indicate that environmental factors (more specifically, the consumption of lactose) may "play a more important role than genetic factors in the etio-pathogenesis of milk intolerance",[11] but some other publications suggest that lactase production does not seem to be induced by dairy/lactose consumption.[3]
[edit] Nutritional concerns
[edit] Primary lactose intolerance
Populations where primary lactose intolerance is the norm have demonstrated similar health levels to westerners (outside of malnutrition issues; see the History of genetic prevalence subsection above) or better health (Japan).
[edit] Secondary lactose intolerance
While secondary lactose intolerance does not inherently affect an individual's nutritional needs, according to accepted medical doctrines in western European and North American countries, dairy is an essential part of a healthy diet. Dairy products are relatively good and accessible sources of calcium and potassium and many countries mandate that milk be fortified with vitamin A and vitamin D. Consequently, in dairy-consuming societies, dairy is often a main source of these nutrients; and, for lacto-vegetarians, a main source of vitamin B12. Individuals who reduce or eliminate consumption of dairy must obtain these nutrients elsewhere. However, Asian populations for whom dairy is not part of their food culture do not present decreased health and sometimes present above average health, like in Japan.
Plant based milk substitutes are not naturally rich in calcium, potassium, or vitamins A or D (and, like most non-animal products, contain no vitamin B12). However, prominent brands are often voluntarily fortified with many of these nutrients.
An increasing number of calcium-fortified breakfast foods, such as orange juice, bread, and dry cereal have been appearing on supermarket shelves. Many fruits and vegetables are rich in potassium and vitamin A; animal products like meat and eggs are rich in vitamin B12, and the human body itself produces some vitamin D from exposure to direct sunlight. Finally, a dietitian or physician may recommend a vitamin or mineral supplement to make up for any remaining nutritional shortfall.
Lactose-reduced dairy products have the same nutritional content as their full-lactose counterparts, but their taste and appearance may differ slightly.
Most infants with gastroenteritis due to rotavirus do not develop lactose intolerance,[58] so these infants do not benefit from being put on a lactose-free diet unless symptoms of lactose intolerance are severe and persistent.
[edit] Congenital lactase deficiency
Congenital lactase deficiency, or CLD, is an autosomal recessive disorder which prevents the expression of lactase.[59] Before the 20th century, infants with this disease rarely survived. As substitute and lactose-free infant formulas later became available, nursing infants affected with CLD could now have their normal nutritional needs met. Beyond infancy, individuals with CLD usually have the same nutritional concerns as those affected by secondary lactose intolerance.
[edit] See also
[edit] References
- Durand, P. (1959). "Lactosurie et saccharosurie". in Ed. E. Rossi, E. Gautier, and J. W. Weber. Paediat. IV. Carbohydrate Metabolism in Children. Basel. pp. 496–502.
- Holzel A, Schwarz V, Sutcliffe KW (1959). "Defective lactose absorption causing malnutrition in infancy". Lancet 1 (7083): 1126–8. doi:. PMID 13665980.
- Carroccio A, Montalto G, Cavera G, Notarbatolo A (1998). "Lactose intolerance and self-reported milk intolerance: relationship with lactose maldigestion and nutrient intake. Lactase Deficiency Study Group". J Am Coll Nutr 17 (6): 631–6. PMID 9853544. http://www.jacn.org/cgi/content/full/17/6/631.
- McGee, Harold (2004). "Milk after infancy: dealing with lactose". On food and cooking: the science and lore of the kitchen. New York: Scribner. pp. 14–15. ISBN 0-684-80001-2.
- Rusynyk RA, Still CD (2001). "Lactose intolerance" (PDF). J Am Osteopath Assoc 101 (4 Suppl Pt 1): S10–2. PMID 11392211. http://www.jaoa.org/cgi/reprint/101/4_suppl_1/10S.
[edit] Footnotes
- ^ "Improved lactose digestion and intolerance among African-American adolescent girls fed a dairy-rich diet.". Journal of the American Dietetic Association. 2000. http://www.accessmylibrary.com/coms2/summary_0286-27939567_ITM. Retrieved on 2009-02-03. "Approximately 75% of the world's population loses the ability to completely digest a physiological dose of lactose after infancy".
- ^ Bulhoes, A. C., et. al. (2007-11). "Correlation between lactose absorption and the C/T-13910 and G/A-22018 mutations of the lactase-phlorizin hydrolase (LCT) gene in adult-type hypolactasia". Brazilian Journal of Medical and Biological Research. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-879X2007001100004&lng=en&nrm=iso&tlng=en. Retrieved on 2008-07-19.
- ^ Heyman MB (2006). "Lactose intolerance in infants, children, and adolescents". Pediatrics 118 (3): 1279–86. doi:. PMID 16951027. http://aappolicy.aappublications.org/cgi/content/full/pediatrics;118/3/1279.
- ^ a b c d e f g h i j k l m Enattah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, Järvelä I (2002). "Identification of a variant associated with adult-type hypolactasia". Nat. Genet. 30 (2): 233–7. doi:. PMID 11788828.
- ^ a b Mark Wiser (August 30, 2007). "Intestinal Protozoa". Tulane University. http://www.tulane.edu/~wiser/protozoology/notes/intes.html. Retrieved on 2008-01-31.
- ^ a b Giardiasis at eMedicine Andre Pennardt February 22, 2006
- ^ Swagerty DL, Walling AD, Klein RM (2002). "Lactose intolerance". Am Fam Physician 65 (9): 1845–50. PMID 12018807. http://www.aafp.org/afp/20020501/1845.html.
- ^ Colic and lactose intolerance
- ^ Soy Nutrition
- ^ Coles Harriet (2007-01-20). "The lactase gene in Africa: Do you take milk?". The Human Genome, Wellcome Trust. http://genome.wellcome.ac.uk/doc_WTX038968.html. Retrieved on 2008-07-18.
- ^ a b c Yoshida Y, Sasaki G, Goto S, Yanagiya S, Takashina K (1975). "Studies on the etiology of milk intolerance in Japanese adults". Gastroenterol. Jpn. 10 (1): 29–34. PMID 1234085.
- ^ a b Flatz G (1987). "Genetics of lactose digestion in humans". Adv. Hum. Genet. 16: 1–77. PMID 3105269.
- ^ a b c d e f g h i j k l m Kretchmer N (1972). "Lactose and lactase". Sci. Am. 227 (4): 71–8. PMID 4672311.
- ^ a b c d e f g h i "Lactose Intolerance: The Molecular Explanation". UC Davis Nutritional Genomics. http://nutrigenomics.ucdavis.edu/nutrigenomics/index.cfm?objectid=968814F6-65B3-C1E7-0C7007B71CC9959A.
- ^ Anne Charlotte Jäger (1, February 2006). "Laktose-intolerans: Gentest for laktose-intolerans - hurtig og billig diagnostik". DSKB-NYT. http://www.dskb.dk/index.jsp?id=7353&gr=7446.
- ^ N.S. Enattah et al. (1, September 2007). "Evidence of Still-Ongoing Convergence Evolution of the Lactase Persistence T-13910 Alleles in Humans". American Journal of Human Genetics. http://www.ajhg.org/AJHG/fulltext/S0002-9297(07)61358-5.
- ^ a b c d e f g h i j k de Vrese M, Stegelmann A, Richter B, Fenselau S, Laue C, Schrezenmeir J (2001). "Probiotics--compensation for lactase insufficiency". Am. J. Clin. Nutr. 73 (2 Suppl): 421S–429S. PMID 11157352. http://www.ajcn.org/cgi/content/full/73/2/421S.
- ^ G.D. Smith et al., Lactase persistence-related genetic variant: population substructure and health outcomes. European Journal of Human Genetics, 2008.
- ^ Valenkevich LN, Iakhontova OI (2005). "[Prevalence of the lactase deficiency among the population of the northwestern region of Russia]" (in Russian). Eksp Klin Gastroenterol (1): 97–100, 108. PMID 15991859.
- ^ a b c Cavalli-Sforza LT, Strata A, Barone A, Cucurachi L (1987). "Primary adult lactose malabsorption in Italy: regional differences in prevalence and relationship to lactose intolerance and milk consumption" (PDF). Am. J. Clin. Nutr. 45 (4): 748–54. PMID 3565303. http://www.ajcn.org/cgi/reprint/45/4/748.pdf.
- ^ http://users.med.up.pt/ruifonte/PDFs/2007-2008/Intol%20Lactose_2007_pag1-14.pdf
- ^ Kozlov A, Lisitsyn D (1997). "Hypolactasia in Saami subpopulations of Russia and Finland". Anthropol Anz 55 (3-4): 281–7. PMID 9468755.
- ^ Jackson RT, Latham MC (1979). "Lactose malabsorption among Masai children of East Africa". Am. J. Clin. Nutr. 32 (4): 779–82. PMID 581925.
- ^ Burgio GR, Flatz G, Barbera C, et al. (1984). "Prevalence of primary adult lactose malabsorption and awareness of milk intolerance in Italy" (PDF). Am. J. Clin. Nutr. 39 (1): 100–4. PMID 6691285. http://www.ajcn.org/cgi/reprint/39/1/100.pdf.
- ^ Vesa TH, Marteau P, Korpela R (2000). "Lactose intolerance". J Am Coll Nutr 19 (2 Suppl): 165S–175S. PMID 10759141. http://www.jacn.org/cgi/content/full/19/suppl_2/165S.
- ^ a b c Wang YG, Yan YS, Xu JJ, et al (1984). "Prevalence of primary adult lactose malabsorption in three populations of northern China". Hum. Genet. 67 (1): 103–6. doi:. PMID 6235167.
- ^ Nasrallah SM (1979). "Lactose intolerance in the Lebanese population and in "Mediterranean lymphoma"" (PDF). Am. J. Clin. Nutr. 32 (10): 1994–6. PMID 484518. http://www.ajcn.org/cgi/reprint/32/10/1994.pdf.
- ^ a b Sahi T (1994). "Genetics and epidemiology of adult-type hypolactasia". Scand. J. Gastroenterol. Suppl. 202: 7–20. doi:. PMID 8042019.
- ^ Woteki CE, Weser E, Young EA (1976). "Lactose malabsorption in Mexican-American children" (PDF). Am. J. Clin. Nutr. 29 (1): 19–24. PMID 946157. http://www.ajcn.org/cgi/reprint/29/1/19.pdf.
- ^ Swallow DM (2003). "Genetics of lactase persistence and lactose intolerance". Annu. Rev. Genet. 37: 197–219. doi:. PMID 14616060.
- ^ Matthews SB, Waud JP, Roberts AG, Campbell AK (2005). "Systemic lactose intolerance: a new perspective on an old problem". Postgrad Med J 81 (953): 167–73. doi:. PMID 15749792.
- ^ R. Bowen (December 28, 2006). "Lactose Intolerance (Lactase Non-Persistence)". Pathophysiology of the Digestive System. Colorado State University. http://www.vivo.colostate.edu/hbooks/pathphys/digestion/smallgut/lactose_intol.html.
- ^ National Digestive Diseases Information Clearinghouse (March 2006). "Lactose Intolerance -- How is lactose intolerance diagnosed?". National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. http://digestive.niddk.nih.gov/ddiseases/pubs/lactoseintolerance/#diagnosed.
- ^ a b Hargrove, James L.; Berdanier, Carolyn D. (1993). Nutrition and gene expression. Boca Raton: CRC Press. ISBN 0-8493-6961-4.
- ^ Wilson J (December 2005). "Milk Intolerance: Lactose Intolerance and Cow's Milk Protein Allergy". Newborn and Infant Nursing Reviews 5 (4): 203–7. doi:.
- ^ "Lactose tolerance/intolerance". Gene Expression. January 19, 2004. http://www.gnxp.com/MT2/archives/001681.html. Retrieved on 2008-01-31.
- ^ Lactose Intolerance from American Family Physician
- ^ Swaminathan, Nikhil (December 11, 2006). "African Adaptation to Digesting Milk Is "Strongest Signal of Selection Ever"". Scientific American. http://www.sciam.com/article.cfm?articleID=727EA883-E7F2-99DF-36D89AB12E930315&sc=I100322.
- ^ Charles Scriver, Beaudet, A.L., Valle, D., Sly, W.S., Vogelstein, B., Childs, B., Kinzler, K.W. (Accessed 2007). The Online Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill. - Summaries of 255 chapters, full text through many universities. There is also the OMMBID blog.
- ^ Lactose Intolerance at eMedicine Roy, Barakat, Nwakakwa, Shojamanesh, Khurana, July 5, 2006 - About 44% of lactose intolerant women regain the ability to digest lactose during pregnancy. This might be caused by slow intestinal transit and intestinal flora changes during pregnancy.
- ^ a b National Digestive Diseases Information Clearinghouse (March 2006). "Lactose Intolerance". National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. http://digestive.niddk.nih.gov/ddiseases/pubs/lactoseintolerance/.
- ^ Composition of Human, Cow, and Goat Milks - Goat Milk - GOATWORLD.COM
- ^ Peeva (2001). "Composition of buffalo milk. Sources of specific effects on the separate components". Bulg. J. Agric. Sci. 7: 329–35. http://bjas.hit.bg/07/693A.htm.
- ^ C:\JAG2\Jiang.vp
- ^ Dairy Science and Technology, Dept. of Food Science. "Ice Cream Formulations". University of Guelph. http://www.foodsci.uoguelph.ca/dairyedu/icform.html.
- ^ Reger, Combs, Coulter and Koch (01 Feb 1951). "A Comparison of Dry Sweet Cream Buttermilk and Non-Fat Dry Milk Solids in Breadmaking". Journal of Dairy Science 34 (2): 136–44. http://jds.fass.org/cgi/content/abstract/34/2/136.
- ^ Goat Milk Composition
- ^ a b "General guidelines for milk allergy". Oregon Health & Science University. http://www.ohsu.edu/health/health-topics/topic.cfm?id=10182.
- ^ "Margarine Regulations". http://www.gov.ns.ca/JUST/REGULATIONS/regs/marge.htm.
- ^ "Enriched White Bread in Canada". The Canadian Celiac Association. http://www.celiac.ca/Articles/PAB%20Enriching%20GF%20Foods.html.
- ^ Riggs, Lloyd K; Beaty, Annabel; Johnson, Arnold H (01 Dec). "Influence of Nonfat Dry Milk Solids on the Nutritive Value of Bread". Journal of Dairy Science 29 (12): 821–9. http://jds.fass.org/cgi/content/abstract/29/12/821.
- ^ "Bartek, food additive company" (PDF). http://www.bartek.ca/pdfs/Applications/SavouryProducts/SavourySnackFoods/Savoury%20Snack%20Foods%20Alphabetical%20List%20of%20Product%20Names.pdf.
- ^ Sahi T. (1974 PMID: 4852638). "Lactose malabsorption in Finnish-speaking and Swedish-speaking populations in Finland". Scand J. Gastroenterol. 9 (3): 303–8. http://www.ncbi.nlm.nih.gov/pubmed/4852638.
- ^ Montalto M, Curigliano V, Santoro L, et al (2006). "Management and treatment of lactose malabsorption". World J. Gastroenterol. 12 (2): 187–91. PMID 16482616. http://www.wjgnet.com/1007-9327/12/187.asp.
- ^ He M, Yang Y, Bian L, Cui H (1999). "[Effect of exogenous lactase on the absorption of lactose and its intolerance symptoms]" (in Chinese). Wei Sheng Yan Jiu 28 (5): 309–11. PMID 12712706.
- ^ O'Connell S, Walsh G (2006). "Physicochemical characteristics of commercial lactases relevant to their application in the alleviation of lactose intolerance". Appl. Biochem. Biotechnol. 134 (2): 179–91. doi:. PMID 16943638.
- ^ Heyman MB (2006). "Lactose intolerance in infants, children, and adolescents". Pediatrics 118 (3): 1279–86. doi:. PMID 16951027. http://pediatrics.aappublications.org/cgi/content/full/118/3/1279.
- ^ Turck D (2007). "[Prevention and treatment of acute diarrhea in infants]" (in French). Arch Pediatr 14 (11): 1375–8. doi:. PMID 17629685.
- ^ Lactose Intolerance at eMedicine Guandalini S, Frye R, Rivera-Hernández D, Miller L, Borowitz S
[edit] External links
- United States National Institutes of Health page regarding lactose intolerance
- Scientific American: African Adaptation to Digesting Milk Is "Strongest Signal of Selection Ever" (East African cattle herding communities rapidly and independently evolved ability to digest lactose)
- Official Lactose Intolerant Informational Website
|
|||||||||||||||||||||||||||||||||||