Lithium-ion battery
From Wikipedia, the free encyclopedia
| Battery specifications | |
 Lithium-ion battery of Varta, Museum Autovision, Altlußheim, Germany |
|
| Energy/weight | 100-160 Wh/kg[1] |
|---|---|
| Energy/size | 250-360 Wh/L[1] |
| Power/weight | ~250-~340 W/kg[2] 1700 W/kg (lab)[3] |
| Charge/discharge efficiency | 80-90%[4] |
| Energy/consumer-price | 2.8-5 Wh/US$[5] |
| Self-discharge rate | 5%-10%/month |
| Time durability | (24-36) months |
| Cycle durability | ~1200 cycles[citation needed] |
| Nominal Cell Voltage | 3.6 / 3.7 V |
Lithium-ion batteries (sometimes abbreviated Li-ion batteries) are a type of rechargeable battery in which a lithium ion moves between the anode and cathode. The lithium ion moves from the anode to the cathode during discharge and in reverse, from the cathode to the anode, when charging.
Lithium ion batteries are common in consumer electronics. They are one of the most popular types of battery for portable electronics, with one of the best energy-to-weight ratios, no memory effect, and a slow loss of charge when not in use. In addition to uses for consumer electronics, lithium-ion batteries are growing in popularity for defense, automotive, and aerospace applications due to their high energy density. However certain kinds of mistreatment may cause Li-ion batteries to explode.
The three primary functional components of a lithium ion battery are the anode, cathode, and electrolyte, for which a variety of materials may be used. Commercially, the most popular material for the anode is graphite. The cathode is generally one of three materials: a layered oxide, such as lithium cobalt oxide, one based on a polyanion, such as lithium iron phosphate, or a spinel, such as lithium manganese oxide, although materials such as TiS2 (titanium disulfide) were originally used.[6] Depending on the choice of material for the anode, cathode, and electrolyte the voltage, capacity, life, and safety of a lithium ion battery can change dramatically. Lithium ion batteries are not to be confused with lithium batteries, the key difference being that lithium batteries are primary batteries containing metallic lithium while lithium-ion batteries are secondary batteries containing an intercalation anode material.
Contents |
[edit] History
Lithium ion batteries were first proposed by M.S. Whittingham (Binghamton University), then at Exxon, in the 1970s.[7] Whittingham used titanium sulfide as the cathode and lithium metal as the anode.
The electrochemical properties of the lithium intercalation in graphite were first discovered in 1982 by Rajeeva R. Agarwal and J. Robert Selman at the Illinois Institute of Technology. They obtained the activity of lithium in graphite and showed the diffusion of lithium was rapid and reversible in essence proving its rechargeability. [8]
Lithium batteries in which the anode is made from metallic lithium pose severe safety issues. As a result, lithium-ion batteries were developed in which the anode, like the cathode, is made of a material containing lithium ions. Lithium-ion batteries came into reality when Bell Labs developed a workable graphite anode[9] to provide an alternative to the (metallic lithium anode) lithium battery. Following groundbreaking cathode research by a team led by John Goodenough[10], the first commercial lithium-ion battery was released by Sony in 1991. The cells used layered oxide chemistry, specifically lithium cobalt oxide. These batteries revolutionized consumer electronics.
In 1983, Michael Thackeray, John Goodenough, and coworkers identified manganese spinel as a cathode material.[11] Spinel showed great promise, since it is a low-cost material, has good electronic and lithium ion conductivity, and possesses a three-dimensional structure which gives it good structural stability. Although pure manganese spinel fades with cycling, this can be overcome with additional chemical modification of the material.[12] Manganese spinel is currently used in commercial cells.[13]
In 1989, Arumugam Manthiram and John Goodenough of the University of Texas at Austin showed that cathodes containing polyanions, eg. sulfates, produce higher voltage than oxides due to the inductive effect of the polyanion.[14]
In late 1996, Padhi, Goodenough and coworkers identified the lithium iron phosphate (LiFePO4) and other phospho-olivines (lithium metal phosphates with olivine-structure) as cathode material for lithium ion batteries.[15] Owing to its tremendous superiority over other cathode materials in terms of cost, safety, stability and performance, LiFePO4 is currently being used or developed for most lithium-ion batteries to power portable devices such as laptop computers and power tools. LiFePO4 is most suitable for large batteries for electric automobiles and other energy storage applications such as load saving, where safety is of utmost importance.
In 2002, Yet-Ming Chiang and his group at MIT published a paper in which they showed a dramatic improvement in the performance of Li batteries by boosting the material's conductivity by doping it with aluminium, niobium and zirconium, though at the time, the exact mechanism causing the increase became the subject of a heated debate.[16]
In 2004, Chiang again increased performance by utilizing iron-phosphate particles less than 100 nanometres across. This miniaturized the particle density by almost 100 fold, increased the surface area of the electrode and improved the battery's ability to store and deliver energy. Commercialization of the iron-phosphate technology led to a competitive market and a patent-infringement battle between Chiang and Goodenough, two of the leading developers of the technology.[16]
[edit] Electrochemistry
The three participants in the electrochemical reactions in a lithium ion battery are the anode, cathode, and electrolyte.
Both the anode and cathode are materials into which and from which lithium can migrate. The process of lithium moving into the anode or cathode is referred to as insertion (or intercalation), and the reverse process, in which lithium moves out of the anode or cathode is referred to as extraction (or deintercalation). When a cell is discharging, the lithium is extracted from the anode and inserted into the cathode. When the cell is charging, the reverse process occurs: lithium is extracted from the cathode and inserted into the anode.
The negative (during discharge) electrode (anode) of a conventional Li-ion cell is made from carbon, the positive (during discharge) electrode (cathode) is a metal oxide, and the electrolyte is a lithium salt in an organic solvent.[17]
Useful work can only be extracted if electrons flow through an external circuit. Therefore the half reactions are enlightening. The following equations are written in units of moles, making it possible to use the coefficient x. The cathode half reaction (with charging being forwards) is: [18]
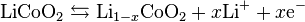
The anode half reaction is:
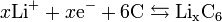
The overall reaction has limits. Overdischarge will supersaturate lithium cobalt oxide, leading to the production of lithium oxide,[19] possibly by the following irreversible reaction:

Overcharge up to 5.2V leads to the synthesis of cobalt(IV) oxide, as evidenced by x-ray diffraction[20]
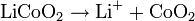
In a lithium-ion battery the lithium ions are transported to and from the cathode or anode, with the transition metal, Co, in LixCoO2 being oxidized from Co3+ to Co4+ during charging, and reduced from Co4+ to Co3+ during discharge.
[edit] Cathodes
| Cathode Material | Average Voltage | Gravimetric Capacity |
|---|---|---|
| LiCoO2 | 3.7 V | 140 mAh/g |
| LiMnO2 | 4.0 V | 100 mAh/g |
| LiFePO4 | 3.3 V | 120 mAh/g |
| Li2FePO4F | 3.6 V | 115 mAh/g |
See uranium trioxide for some details of how the cathode works. While uranium oxides are not used in commercially made batteries, the way in which uranium oxides can reversibly insert cations is the same as the way in which the cathode in many lithium-ion cells work.[citation needed]
[edit] Electrolytes
The cell voltages above are larger than the potential at which aqueous solutions electrolyze. Therefore, nonaqueous solutions are used.
Liquid electrolytes in Li-ion batteries consist of lithium salts, such as LiPF6, LiBF4, or LiClO4, in an organic solvent, such as ether. A liquid electrolyte conducts Li ions, acting as a carrier between the cathode and the anode when a battery passes an electric current through an external circuit. Typical conductivities of liquid electrolyte at room temperature (20 oC) are in the range of 10 mS/cm, increasing by approximately 30-40% at 40 oC and decreasing by a slightly smaller amount at 0 oC.[21]
Unfortunately, organic solvents are easily decomposed on anodes during charging. However, when appropriate organic solvents are used as the electrolyte, the solvent is decomposed and forms a solid layer called the solid electrolyte interphase (SEI)[22] at first charge that is electrically insulating yet sufficiently conductive to lithium ions. The interphase prevents decomposition of the electrolyte after the second charge. For example, ethylene carbonate is decomposed at a relatively high voltage, 0.7 V vs. Li, and forms a dense and stable interface.[citation needed]
[edit] Advantages and disadvantages
[edit] Advantages
Lithium-ion batteries can be formed into a wide variety of shapes and sizes so as to efficiently fill available space in the devices they power.
Li-ion batteries are lighter than other equivalent secondary batteries—often much lighter. The energy is stored in these batteries through the movement of lithium ions. However, the bulk of the electrodes are effectively "housing" for the ions and add weight, and in addition "dead weight" from the electrolyte, current collectors, casing, electronics and conductivity additives reduce the charge per unit mass to little more than that of other rechargeable batteries. A key advantage of using Li-ion chemistry is the high open circuit voltage that can be obtained in comparison to aqueous batteries (such as lead acid, nickel metal hydride and nickel cadmium).[citation needed]
Li-ion batteries do not suffer from the memory effect. They also have a low self-discharge rate of approximately 5% per month, compared with over 30% per month in common nickel metal hydride batteries (Low self-discharge NiMH batteries have much lower values, around 1.25% per month) and 10% per month in nickel cadmium batteries.
According to one manufacturer, Li-ion cells (and, accordingly, "dumb" Li-ion batteries) do not have any self-discharge in the usual meaning of this word.[18] What looks like a self-discharge in these batteries is a permanent loss of capacity, described in more detail below. On the other hand, "smart" Li-ion batteries do self-discharge, due to the small constant drain of the built-in voltage monitoring circuit. This drain is the most important source of self-discharge in these batteries.
[edit] Disadvantages of traditional Li-ion technology
| This section needs additional citations for verification. Please help improve this article by adding reliable references (ideally, using inline citations). Unsourced material may be challenged and removed. (October 2007) |
[edit] Shelf life
A unique drawback of the Li-ion battery is that its service life is dependent upon aging (shelf life). From time of manufacturing, regardless of whether it was charged or the number of charge/discharge cycles, the battery will decline slowly and predictably in "capacity". This means an older battery will not last as long as a new battery due solely to its age, unlike other batteries. This is due to an increase in internal resistance, which affects its ability to deliver current, thus the problem is more pronounced in high-current applications than low. This drawback is not widely published.[23] However, as this capacity decreases over time, the time required to charge it also decreases proportionally. Also, high charge levels and elevated temperatures hasten permanent capacity loss for Lithium ion batteries.[24] This heat is caused by the traditional carbon anode, which has been replaced with good results by Lithium titanate. Lithium titanate has been experimentally shown to drastically reduce the degenerative effects associated with charging including expansion and other factors.[25] See "Improvements of lithium Ion technology" below.
At a 100% charge level, a typical Li-ion laptop battery that is full most of the time at 25 °C or 77 °F will irreversibly lose approximately 20% capacity per year. However, a battery in a poorly ventilated laptop may be subject to a prolonged exposure to much higher temperatures, which will significantly shorten its life. Different storage temperatures produce different loss results: 6% loss at 0 °C (32 °F), 20% at 25 °C (77 °F), and 35% at 40 °C (104 °F). When stored at 40%–60% charge level, the capacity loss is reduced to 2%, 4%, 15% at 0, 25 and 40 degrees Celsius respectively.[26]
[edit] High internal resistance
The internal resistance of lithium-ion batteries is high compared to other rechargeable chemistries such as nickel-metal hydride and nickel-cadmium. The internal resistance of a typical lithium-ion cell is around 320 mOhm when new, compared to less than 100 mOhm for a NiCd cell, and it increases with both cycling and chronological age.[27] Rising internal resistance causes the voltage at the terminals to drop under load, reducing the maximum current that can be drawn from them. Eventually they reach a point at which the battery can no longer operate the equipment it is installed in for an adequate period.
High drain applications such as power tools may require the battery to be able to supply a current as high as (15 h-1)C (that is, a current level that would drain the battery in 1/15 hour if sustained; e.g. 22.5 A for a battery with a capacity of 1.5 Ah). Lower-power devices such as MP3 players may only require (0.1 h-1)C (e.g. 150 mA for a battery with a capacity of 1500 mAh). With similar battery technology, the MP3 player's battery will effectively last much longer, since it can tolerate a much higher internal resistance.[28]
[edit] Protection circuits required
Li-ion batteries are not as durable as nickel metal hydride or nickel-cadmium designs, and can be extremely dangerous if mistreated. They may explode if overheated or if charged to an excessively high voltage. Furthermore, they may be irreversibly damaged if discharged below a certain voltage. To reduce these risks, li-ion batteries generally contain a small circuit that shuts down the battery when discharged below a certain threshold (typically 3 V) or charged above a certain limit (typically 4.2 V).
This circuit prevents deep discharge in normal use. However, when stored for long periods, the small current drawn by the protection circuitry may deeply drain the battery. Some applications attempt to recover deeply discharged cells by slow-charging them.
Furthermore, this circuit adds to the cost of lithium-ion batteries, which is usually higher than that of comparable-capacity NiMH or NiCD batteries.
[edit] Reserves
The lithium reserves are estimated at 30 million tonnes in 2015[29].
[edit] Safety features
Li-ion chemistry is not as safe as nickel metal hydride or nickel-cadmium, and a li-ion cell requires several mandatory safety devices to be built in before it can be considered safe for use outside of a laboratory. These are:[18]
- shut-down separator (for overtemperature),
- tear-away tab (for internal pressure),
- vent (pressure relief), and
- thermal interrupt (overcurrent/overcharging).
These devices occupy useful space inside the cells, and reduce their reliability; typically, they permanently and irreversibly disable the cell when activated. They are required because the anode produces heat during use, while the cathode may produce oxygen during use. Safety devices and recent, improved electrode designs greatly reduce or eliminate the risk of fire or explosion.
The safety features of lithium-ion cells can be compared with nickel metal hydride cells, which only have a hydrogen/oxygen recombination device (preventing damage due to mild overcharging) and a back-up pressure valve.[citation needed]
[edit] Product recalls
About 1% of Li-ion batteries are the subject of recalls.[30] .
[edit] Specifications and design
- Specific energy density: 150 to 200 Wh/kg (540 to 720 kJ/kg)
- Volumetric energy density: 250 to 530 Wh/l (900 to 1900 J/cm³)
- Specific power density: 300 to 1500 W/kg (@ 20 seconds[31] and 285 Wh/l)
Because lithium-ion batteries can have a variety of cathode and anode materials, the energy density and voltage vary from chemistry to chemistry.
Lithium ion batteries with a lithium iron phosphate cathode and graphite anode have a nominal open-circuit voltage of 3.2 V and a typical charging voltage of 3.6 V. Lithium nickel manganese cobalt (NMC) oxide cathode with graphite anodes have a 3.7 V nominal voltage with a 4.2 V max charge. The charging procedure is done at constant voltage with current limiting circuitry. This means charging with constant current until a voltage of 4.2 V is reached by the cell and continuing with a constant voltage applied until the current drops close to zero. Typically the charge is terminated at 7% of the initial charge current. In the past, lithium-ion batteries could not be fast-charged and typically needed at least two hours to fully charge. Current generation cells can be fully charged in 45 minutes or less; some Lithium-Ion variants can reach 90% in as little as 10 minutes.[32]
[edit] Improvements to Lithium Ion Battery Technology
[edit] Overview
Improvements focus on several areas, and often involve advances in nanotechnology and microstructures:
- Increasing cycle life and performance (decreases internal resistance and increases output power) by changing the composition of the material used in the anode and cathode along with increasing the effective surface area of the electrodes.(related developments have helped ultracapacitors )
- Improving capacity by improving the structure to incorporate more active materials.
- Improving the safety of Lithium Ion style batteries.
[edit] Manganese Spinel Cathodes
LG, which is the third largest producer of lithium ion batteries, uses the lithium manganese spinel for its cathode. It is working with its subsidiary CPI to commercialize lithium ion batteries containing manganese spinel for HEV applications.[33] Several other companies are also working on manganese spinel, including NEC and Samsung.[34]
[edit] Lithium Iron Phosphate Cathode With Traditional Anode
The University of Texas first licensed its patent for lithium iron phosphate cathodes to the canadian utility Hydro-Québec.[35] Phostech was later spun-off from Hydro-Québec for the sole development of lithium iron phosphate.
Valence Technology, located in Austin, Texas, is also working on lithium iron phosphate cells. Since March 2005, the Segway Personal Transporter has been shipping with extended-range lithium-ion batteries[36] made by Valence Technology using iron phosphate cathode materials. Segway, Inc. chose to build their large-format battery with this cathode material because of its improved safety over metal-oxide materials.
In November 2005, A123Systems announced[37] the development of lithium iron phosphate cells based on research licensed from MIT.[38][39] While the battery has slightly lower energy density than other competing Lithium Ion technologies, a 2 Ahr cell can provide a peak of 70 Amps without damage and operate at temperatures above 60 degrees C. Their first cell is in production (1Q/2006) and being used in consumer products including DeWalt power tools, aviation products, automotive hybrid systems and PHEV conversions.
LiFePO4 cells are currently available commercially.
[edit] High Power Cathode using Lithium Nickel Manganese Cobalt (NMC)
Imara Corporation, based out of Menlo Park, CA is commercializing a new materials-agnostic technology first applied on an NMC material which has the effect of lowering impedance and extending cycle life. These high power capable cells have high energy density relative to other high power cells in the market.[40] The batteries are being deployed in power tools, outdoor power equipment and hybrid vehicles.
[edit] Traditional Cathode With Lithium Titanate Anode
Altairnano, a small firm based in Reno, Nevada, has announced a nano-sized titanate electrode material for lithium-ion batteries. It is claimed the prototype battery has three times the power output of existing batteries and can be fully charged in six minutes. However the energy capacity is about half that of normal li-ion cells. The company also says the battery cells have now achieved a life of over 9,000 charge cycles and they still retain up to 85% charge capacity, so durability and battery life are much longer, estimated to be around 20 years or four times longer than regular lithium-ion batteries. The batteries can operate from -50 °C to over 75 °C and will not explode or result in thermal runaway even under severe conditions because they do not contain graphite-coated-metal anode electrode material.[41] The batteries are currently being tested in a new production car made by Phoenix Motorcars which was on display at the 2006 SEMA motorshow. They're also being tested, on a one megawatt grid scale, in the PJM Interconnection Regional Transmission Organization control area[42] in Norristown, PA as well as by several branches of the U.S. Department of Defense.[43] In addition the batteries are being demonstrated by Proterra in their all-electric EcoRide BE35 vehicle, a lightweight 35-foot bus.[44] Altairnano is currently working with three different cell chemistries, for various energy and power storage applications, with another new cell chemistry expected in the fall of 2009. The nature of their latest cathode materials is currently proprietary.
[edit] Combined anode and cathode developments
EnerDel, which is jointly owned by Ener1 and Delphi, is working to commercialize cells containing a titanate anode and manganese spinel cathode.[45] Although the cells show excellent thermal properties and cyclability, their low voltage may hamper commercial success.[46]
[edit] Research Claims
In April 2006, a group of scientists at MIT announced a process which uses viruses to form nano-sized wires. These can be used to build ultrathin lithium-ion batteries with three times the normal energy density.[47]
As of June 2006, researchers in France have created nanostructured battery electrodes with several times the energy capacity, by weight and volume, of conventional electrodes.[48]
In the September 2007 issue of Nature, researchers from the University of Waterloo, Canada, reported a new cathode chemistry, whereby the hydroxide group in the iron phosphate cathode was replaced by fluorine. [5] The advantages seem to be two-fold. First, there is less volume change in the cathode over a charge cycle which indicates a possibility for longer battery life. Second, the chemistry allows the substitution of Sodium or a Sodium/Lithium mixture for the Lithium in the battery (hence their reference to it as an Alkali-Ion battery).
In November 2007, Subaru unveiled their concept G4e electric vehicle with a lithium vanadium oxide based lithium ion battery, promising double the energy density of a conventional lithium ion battery (lithium cobalt oxide and graphite).[6] In the lab, Lithium vanadium oxide anodes, paired with lithium cobalt oxide cathodes, have achieved 745Wh/l, nearly three times the volumetric energy density of conventional lithium ion batteries. [7]
In December 2007, researchers at Stanford University reported creating a lithium ion nanowire battery with ten times the energy density (amount of energy available by weight) through using silicon nanowires deposited on stainless steel as the anode. The battery takes advantage of the fact that silicon can hold large amounts of lithium, and helps alleviate the longstanding problem of cracking by the small size of the wires. [8] To gain a tenfold improvement in energy density, the cathode would need to be improved as well; however, just improving the anode as such could provide "several" times the energy density, according to the team. The team leader, Yi Cui, expects to be able to commercialize the technology in about five years.[9]. Having a large capacitive anode won't increase the capacity of the battery as predicted by the author when the cathode material is far less capacitive than the anode. However current Li-ion capacity is mainly limited by the low theoretical capacity (372 mAh g−1) of graphite as the anode material so improvement would be significant and limited by cathode material instead of anode.
There are trials in progress to use metal hydrides as anode material for Li-ion batteries. Practical capacity of electrode as high as 1480 mAh g-1 is reported.[10]
[edit] Guidelines for prolonging Li-ion battery life
- Like many rechargeable batteries, lithium-ion batteries should be charged early and often. However, if they are not used for a long time, they should be brought to a charge level of around 40%–60%
- Lithium-ion batteries should not be frequently fully discharged and recharged ("deep-cycled"), but this may be necessary after about every 30th recharge to recalibrate any electronic charge monitor (e.g. a battery meter). This allows the monitoring electronics to more accurately estimate battery charge.[28]
- Li-ion batteries should never be depleted to below their minimum voltage, 2.4 V to 3.0 V per cell.
- Li-ion batteries should be kept cool. Ideally they are stored in a refrigerator. Aging will take its toll much faster at high temperatures. The high temperatures found in cars cause lithium-ion batteries to degrade rapidly.
- Li-ion batteries should not be frozen [49] (most lithium-ion battery electrolytes freeze at approximately −40 °C; however, this is much colder than the lowest temperature reached by household freezers).
- Li-ion batteries should be bought only when needed, because the aging process begins as soon as the battery is manufactured.[28]
- When using a notebook computer running from fixed line power over extended periods, consider removing the battery[50] and storing it in a cool place so that it is not affected by the heat produced by the computer.
[edit] Storage temperature and charge
Storing a Li-ion battery at the correct temperature and charge makes all the difference in maintaining its storage capacity. The following table shows the amount of permanent capacity loss that will occur after storage at a given charge level and temperature.
| Storage Temperature | 40% Charge | 100% Charge |
|---|---|---|
| 0 °C (32 °F) | 2% loss after 1 year | 6% loss after 1 year |
| 25 °C (77 °F) | 4% loss after 1 year | 20% loss after 1 year |
| 40 °C (104 °F) | 15% loss after 1 year | 35% loss after 1 year |
| 60 °C (140 °F) | 25% loss after 1 year | 80% loss after 6 months |
| Source: BatteryUniversity.com[28] | ||
It is significantly beneficial to avoid storing a lithium-ion battery at full charge. A Li-ion battery stored at 40% charge will last many times longer than one stored at 100% charge, particularly at higher temperatures.[28]
If a Li-ion battery is stored with too low a charge, there is a risk of allowing the charge to drop below the battery's low-voltage threshold, resulting in an unrecoverable dead battery. Once the charge has dropped to this level, recharging it can be dangerous. Some batteries therefore feature an internal safety circuit which will prevent charging in this state, and the battery will be for all practical purposes dead.[citation needed]
In circumstances where a second Li-ion battery is available for a given device, it is recommended that the unused battery be discharged to 40% and placed in the refrigerator to prolong its shelf life. While the battery can be used or charged immediately, some Li-ion batteries will provide more energy when brought to room temperature.
[edit] Prolonging Life in Multiple Cells Through Cell Balancing
Analog front ends that balance cells and eliminate mismatches of cells in series or parallel significantly improve battery efficiency and increase the overall pack capacity. As the number of cells and load currents increase, the potential for mismatch also increases. There are two kinds of mismatch in the pack: State-of-Charge (SOC) and capacity/energy (C/E) mismatch. Though the SOC mismatch is more common, each problem limits the pack capacity (mAh) to the capacity of the weakest cell.
It is important to recognize that the cell mismatch results more from limitations in process control and inspection than from variations inherent in the Lithium Ion chemistry. The use of cell balancing can improve the performance of series connected Li-ion Cells by addressing both SOC and C/E issues.[51] SOC mismatch can be remedied by balancing the cell during an initial conditioning period and subsequently only during the charge phase. C/E mismatch remedies are more difficult to implement and harder to measure and require balancing during both charge and discharge periods.
- Cell Balancing
Cell balancing is defined as the application of differential currents to individual cells (or combinations of cells) in a series string. Normally, of course, cells in a series string receive identical currents. A battery pack requires additional components and circuitry to achieve cell balancing. However, the use of a fully integrated analog front end for cell balancing[52] reduces the required external components to just balancing resistors.
This type of solution eliminates the need for discrete capacitors, diodes and most other resistors to achieve balance.
Battery pack cells are balanced when all the cells in the battery pack meet two conditions:
- If all cells have the same capacity, then they are balanced when they have the same State of Charge (SOC.) In this case, the Open Circuit Voltage (OCV) is a good measure of the SOC. If, in an out of balance pack, all cells can be differentially charged to full capacity (balanced), then they will subsequently cycle normally without any additional adjustments. This is mostly a one shot fix.
- If the cells have different capacities, they are also considered balanced when the SOC is the same. But, since SOC is a relative measure, the absolute amount of capacity for each cell is different. To keep the cells with different capacities at the same SOC, cell balancing must provide differential amounts of current to cells in the series string during both charge and discharge on every cycle.
[edit] Safety
Lithium-ion batteries can rupture, ignite, or explode when exposed to high temperature environments, for example in an area that is prone to prolonged direct sunlight.[53] Short-circuiting a Li-ion battery can cause it to ignite or explode, and as such, any attempt to open or modify a Li-ion battery's casing or circuitry is dangerous. Li-ion batteries contain safety devices that protect the cells inside from abuse, and, if damaged, can cause the battery to ignite or explode.
Contaminants inside the cells can defeat these safety devices. For example, the mid-2006 recall of approximately 10 million Sony batteries used in Dell, Sony, Apple, Lenovo/IBM, Panasonic, Toshiba, Hitachi, Fujitsu and Sharp laptops was stated to be as a consequence of internal contamination with metal particles. Under some circumstances, these can pierce the separator, causing the cell to short, rapidly converting all of the energy in the cell to heat resulting in an exothermic oxidizing reaction, increasing the temperature to a few hundred degrees Celsius in a fraction of a second.[54] This causes the neighboring cells to heat up, causing a chain thermal reaction.
The mid-2006 Sony laptop battery recall was not the first of its kind, however it was the largest to date. During the past decade there have been numerous recalls of lithium-ion batteries in cellular phones and laptops owing to overheating problems. In October 2004, Kyocera Wireless recalled approximately 1 million batteries used in cellular phones, due to counterfeit batteries produced in Kyocera's name.[55] In December 2006, Dell recalled approximately 22,000 batteries from the U.S. market.[56] In March 2007, Lenovo recalled approximately 205,000 9-cell lithium-ion batteries due to an explosion risk. In August 2007, Nokia recalled over 46 million lithium-ion batteries, warning that some of them might overheat and possibly explode.[57] There was an incident in the Philippines involving a Nokia N91, which uses the BL-5C battery.[58]
Replacing the lithium cobalt oxide cathode material in li-ion batteries with lithiated metal phosphate leads to longer cycle, and shelf life, improves safety, but lowers capacity. Currently these 'safer' li-ion batteries are mainly used in electric cars and other large-capacity battery applications, where the safety issues are more critical. [59]
Another option is to use manganese oxide or iron phosphate cathode.[60]
A new class of high power cathode materials, lithium nickel manganese cobalt (NMC) oxide has recently been introduced that have 40% higher energy density than iron phosphate, 3 times the cycle life of manganese oxide and exceptional high power performance. These cells have a significantly higher temperature tolerance compared to lithium cobalt oxide. [61]
[edit] Restrictions on transportation
As of January 2008, the US Department of Transportation issued a new rule that permits passengers on board commercial aircraft to carry lithium batteries in their checked baggage, if and only if they are installed in a device.[62].
The purpose of this restriction is that it greatly reduces the chances of them becoming short-circuited and causing a fire. The only types of batteries affected by this rule are ones containing lithium, including Li-ion, Lithium Polymer, and Lithium Cobalt Oxide chemistries. Incidentally, hand luggage is not affected by the ruling.
[edit] Uses
Across the globe, major automakers including General Motors,BYD Auto, Hyundai, Toyota, and Tata Motors have been racing to be first to sell electric vehicles powered by lithium-ion batteries.[63] A US company Tesla Motors is already delivering their lithium-ion-powered BEV Tesla Roadster.[64] In the aftermarket, models such as the Prius have been fitted with lithium-ion batteries.[65]
[edit] See also
- Battery balancer
- Battery holder
- Battery recycling
- Lithium battery
- Lithium iron phosphate battery
- Nanowire battery
- Power-to-weight ratio
- Silver-oxide battery
- Superpolymer
- Lithium polymer battery
[edit] References
- ^ a b [1]
- ^ http://www.panasonic.com/industrial/battery/oem/images/pdf/Panasonic_LiIon_CGA103450A.pdf
- ^ Lithium Ion Battery Research
- ^ The effect of PHEV and HEV duty cycles on battery and battery pack performance, http://www.pluginhighway.ca/PHEV2007/proceedings/PluginHwy_PHEV2007_PaperReviewed_Valoen.pdf
- ^ http://www.werbos.com/E/WhoKilledElecPJW.htm (which links to http://www.thunder-sky.com/home_en.asp)
- ^ MRS Website : Theme Article - Science and Applications of Mixed Conductors for Lithium Batteries
- ^ Electrical Energy Storage and Intercalation Chemistry - WHITTINGHAM 192 (4244): 1126 - Science
- ^ Activity and Diffusivity of Lithium Intercalated in Graphite - Dept. of Energy Citation
- ^ US patent 4304825, "Rechargeable battery", granted 1981-12-08
- ^ USPTO search for inventions by "Goodenough, John"
- ^ http://dx.doi.org/10.1016/0025-5408(83)90138-1
- ^ http://books.google.com/books?id=k4duxuea3eIC
- ^ IEEE Spectrum: Lithium Batteries Take to the Road
- ^ http://dx.doi.org/10.1016/0378-7753(89)80153-3
- ^ Phospho-olivines as positive-electrode materials for rechargeable lithium batteries, A.K. Padhi, K.S. Nanjundaswamy and J.B. Goodenough, J. Electrochem. Soc., 144, 1188-1194 (1997).. http://scitation.aip.org/getabs/servlet/GetabsServlet?prog=normal&id=JESOAN000144000004001188000001&idtype=cvips&gifs=yes.
- ^ a b "In search of the perfect battery". The Economist. 2008-03-06. http://www.economist.com/science/tq/displaystory.cfm?story_id=10789409. Retrieved on 2008-08-24.
- ^ Silberberg, M. 2006. Chemistry: The Molecular Nature of Matter and Change, 4th Ed. New York (NY): McGraw-Hill Education. p 935.
- ^ a b c (pdf)Gold Peak Industries Ltd., Lithium Ion technical handbook. http://www.gpbatteries.com/html/pdf/Li-ion_handbook.pdf.
- ^ H.C. Choi et al., J. Phys. Chem. B 107 p5806(2003) doi:10.1021/jp030438w
- ^ G.G. Amatucci, J.M. Tarascon, L.C. Kein J. Electrochemical Society 143 p1114 1996 doi:10.1149/1.1836594
- ^ http://www.cheric.org/PDF/Symposium/S-J2-0063.pdf
- ^ Balbuena, P.B., Wang, Y.X., eds. Lithium Ion Batteries: Solid Electrolyte Interphase 2004 Imperial College Press, London
- ^ Buchmann, Isidor. "Will Lithium-Ion batteries power the new millennium?". Isidor Buchmann (CEO of Cadex Electronics Inc.). http://www.buchmann.ca/Article5-Page1.asp.
- ^ Aging - capacity loss
- ^ Altair Nano: Power & Energy Systems
- ^ Battery University: Fig. 1 Non-recoverable capacity loss
- ^ Buchmann, Isidor. "Choosing a battery that will last". Isidor Buchmann (CEO of Cadex Electronics Inc.). http://www.buchmann.ca/Article9-Page1.asp.
- ^ a b c d e Buchmann, Isidor (September 2006). "BatteryUniversity.com: How to prolong lithium-based batteries". Cadex Electronics Inc.. http://www.batteryuniversity.com/parttwo-34.htm.
- ^ Pag4.- The trouble with lithium
- ^ Lewis, Leo (August 21, 2007). "Japanese experts demand change to make phones and laptops safe". The Times. http://business.timesonline.co.uk/tol/business/industry_sectors/technology/article2295743.ece.
- ^ http://www.e-one.com.tw/News_2005_e.htm
- ^ AeroVironment Achieves Electric Vehicle Fast Charge Milestone Test Rapidly Recharges a Battery Pack Designed for Use in Passenger Vehicles. 10 Minute Re-Charge Restores Enough Energy to Run Electric Vehicle for Two Hours at 60 Miles Per Hour
- ^ http://www.aei-online.org/automag/techbriefs/10-2006/1-114-10-16.pdf
- ^ IEEE Spectrum: Lithium Batteries Take to the Road
- ^ Bickel & Brewer - A law firm devoted exclusively to the resolution of complex commercial disputes. Bickel & Brewer was formed with a singular goal: to serve clients in significant, disputed matters that involve substantial dollar or business exposures...: Detail
- ^ http://www.segway.com/personal-transporter/lithium_ion.html
- ^ http://www.a123systems.com/html/news/articles/051102_news.html
- ^ Green Car Congress: A123Systems Launches New Higher-Power, Faster Recharging Li-Ion Battery Systems
- ^ Yahoo! Groups
- ^ [http://www.imaracorp.com
- ^ Microsoft PowerPoint - 061125 Altair EDTA Presentation
- ^ [2]
- ^ [3]
- ^ [4]
- ^ Welcome to Ener1
- ^ Microsoft PowerPoint - EnerDel Technical Presentation.ppt [Read-Only]
- ^ Science Express (preprint) http://www.sciencemag.org/cgi/content/abstract/1122716
- ^ Technology Review: Higher-Capacity Lithium-Ion Batteries
- ^ L.M. Cristo, T. B. Atwater. Characteristics and Behavior of 1M LiPF6 1EC:1DMC Electrolyte at Low Temperatures. Fort Monmouth, NJ: U.S. Army Research.
- ^ How to prolong lithium-based batteries
- ^ AN1333
- ^ ISL9208 Multi-Cell Li-ion Battery Pack OCP/Analog Front End
- ^ http://www.tayloredge.com/museum/mymuseum/sciencefun/li-ion_003.mov
- ^ Dell laptop explodes at Japanese conference - The INQUIRER
- ^ Kyocera Wireless (2004-10-28). Kyocera Launches Precautionary Battery Recall, Pursues Supplier of Counterfeit Batteries. Press release. http://www.kyocera-wireless.com/news/20041028_2.htm.
- ^ Tullo, Alex. "Dell Recalls Lithium Batteries." Chemical and Engineering News 21 August 2006: 11.
- ^ http://en.wikinews.org/wiki/Nokia_issues_BL-5C_battery_warning%2C_offers_replacement
- ^ Nokia N91 cell phone explodes
- ^ Safety Last - New York Times
- ^ Technology Review: New Batteries Readied for GM's Electric Vehicle
- ^ http://www.imaracorp.com
- ^ http://www.atbatt.com/blog/31.asp
- ^ http://www.cleantech.com/news/3694/electrovaya-tata-motors-make-electric-indica
- ^ http://www.teslamotors.com
- ^ http://www.calcars.org/priusplus.html
[edit] External links
- Charging the Lithium Ion Battery.
- How Lithium-ion Batteries Work.
- Stanford's nanowire battery holds 10 times the charge of existing ones, Stanford Report, December 18, 2007
- The Lithium Ion Battery.
- Advantages and Limitations of the Lithium Polymer Battery, Jan 2007.



