Ethanol
From Wikipedia, the free encyclopedia
| Ethanol | |
|---|---|
| IUPAC name |
|
| Other names | Ethyl alcohol; grain alcohol; pure alcohol; hydroxyethane; drinking alcohol; ethyl hydrate |
| Identifiers | |
| CAS number | 64-17-5 |
| RTECS number | KQ6300000 |
| SMILES |
|
| ChemSpider ID | |
| Properties | |
| Molecular formula | C2H6O |
| Molar mass | 46.07 g mol−1 |
| Appearance | colorless clear liquid |
| Density | 0.789 g/cm3 |
| Melting point |
−114.3 °C, 159 K, -174 °F |
| Boiling point |
78.4 °C, 352 K, 173 °F |
| Solubility in water | Fully miscible |
| Acidity (pKa) | 15.9 |
| Viscosity | 1.200 mPa·s (cP) at 20.0 °C |
| Dipole moment | 5.64 fC·fm (1.69 D) (gas) |
| Hazards | |
| MSDS | External MSDS |
| EU classification | |
| NFPA 704 | |
| R-phrases | R11 R20 R21 R22 R36 |
| S-phrases | (S2) S7 S16 |
| Flash point | 286.15 K (13 °C or 55.4 °F) |
| Related compounds | |
| Related compounds | methanol, propanol |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug, best known as the type of alcohol found in alcoholic beverages and in modern thermometers. Ethanol is one of the oldest recreational drugs known to man. In common usage, it is often referred to simply as alcohol or spirits.
Ethanol is a straight-chain alcohol, and its molecular formula is C2H5OH. Its empirical formula is C2H6O. An alternative notation is CH3-CH2-OH, which indicates that the carbon of a methyl group (CH3-) is attached to the carbon of a methylene group (-CH2-), which is attached to the oxygen of a hydroxyl group (-OH). It is a constitutional isomer of dimethyl ether. Ethanol is often abbreviated as EtOH, using the common organic chemistry notation of representing the ethyl group (C2H5) with Et.
The fermentation of sugar into ethanol is one of the earliest organic reactions employed by humanity. The intoxicating effects of ethanol consumption have been known since ancient times. In modern times, ethanol intended for industrial use is also produced from by-products of petroleum refining.[1]
Ethanol has widespread use as a solvent of substances intended for human contact or consumption, including scents, flavorings, colorings, and medicines. In chemistry, it is both an essential solvent and a feedstock for the synthesis of other products. It has a long history as a fuel for heat and light and also as a fuel for internal combustion engines.
Contents |
[edit] History
Ethanol has been used by humans since prehistory as the intoxicating ingredient of alcoholic beverages. Dried residues on 9,000-year-old pottery found in China imply that alcoholic beverages were used even among Neolithic people.[2] Its isolation as a relatively pure compound was first achieved by the Persian alchemist, Muhammad ibn Zakarīya Rāzi (Rhazes, 865–925).
Two other chemists who contributed to the development of distillation techniques were Geber (Jabir ibn Hayyan) and Al-Kindi (Alkindus). Writings attributed to Geber (721–815) mention the flammable vapors of boiled wine. Al-Kindi (801–873) unambiguously described the distillation of wine.[3]
In 1796, Johann Tobias Lowitz obtained pure ethanol by filtering distilled ethanol through activated charcoal.
Antoine Lavoisier described ethanol as a compound of carbon, hydrogen, and oxygen, and in 1808 Nicolas-Théodore de Saussure determined ethanol’s chemical formula.[4] Fifty years later, Archibald Scott Couper published the structural formula of ethanol, which placed ethanol among the first compounds whose chemical structure had been determined.[5]
Ethanol was first prepared synthetically in 1826 through the independent efforts of Henry Hennel in Great Britain and S.G. Sérullas in France. In 1828, Michael Faraday prepared ethanol by acid-catalyzed hydration of ethylene, a process similar to that which is used today for industrial ethanol synthesis.[6]
Ethanol was used as lamp fuel in the United States as early as 1840, but a tax levied on industrial alcohol during the Civil War made this use uneconomical. This tax was repealed in 1906,[7] and from 1908 onward Ford Model T automobiles could be adapted to run on ethanol.[8] With the advent of Prohibition in 1920 though, sellers of ethanol fuel were accused of being allied with moonshiners,[7] and ethanol fuel again fell into disuse until late in the 20th century.
[edit] Physical properties
Ethanol is a volatile, colorless liquid that has a strong characteristic odor. It burns with a smokeless blue flame that is not always visible in normal light.
The physical properties of ethanol stem primarily from the presence of its hydroxyl group and the shortness of its carbon chain. Ethanol’s hydroxyl group is able to participate in hydrogen bonding, rendering it more viscous and less volatile than less polar organic compounds of similar molecular weight.
Ethanol is a versatile solvent, miscible with water and with many organic solvents, including acetic acid, acetone, benzene, carbon tetrachloride, chloroform, diethyl ether, ethylene glycol, glycerol, nitromethane, pyridine, and toluene.[9][10] It is also miscible with light aliphatic hydrocarbons, such as pentane and hexane, and with aliphatic chlorides such as trichloroethane and tetrachloroethylene.[10]
Ethanol’s miscibility with water contrasts with that of longer-chain alcohols (five or more carbon atoms), whose water miscibility decreases sharply as the number of carbons increases.[11] The miscibility of ethanol with alkanes is limited to alkanes up to undecane, mixtures with dodecane and higher alkanes show a miscibility gap below a certain temperature (approx. 13 °C for dodecane[12]). The miscisbility gap tends to get wider with higher alkanes and the temperature for complete miscibility increases.
Ethanol-water mixtures have less volume than the sum of their individual components at the given fractions. Mixing equal volumes of ethanol and water results in only 1.92 volumes of mixture.[9][13] Mixing ethanol and water is exothermic. At 298 K up to approx. 777 J/mol[14] are set free.
Mixtures of ethanol and water form an azeotrope at approx. 89 mole-% ethanol and 11 mole-% water[15] or a mixture of about 96 volume percent ethanol and 4 % water at normal pressure and T=351 K. This azeotropic composition is strongly temperature- and pressure-dependent and vanishes at temperatures below 303 K[16].
|
Vapor-liquid equilibrium of the mixture of Ethanol and Water (incl. azeotrope) |
Hydrogen bonding causes pure ethanol to be hygroscopic to the extent that it readily absorbs water from the air. The polar nature of the hydroxyl group causes ethanol to dissolve many ionic compounds, notably sodium and potassium hydroxides, magnesium chloride, calcium chloride, ammonium chloride, ammonium bromide, and sodium bromide.[10] Sodium and potassium chlorides are slightly soluble in ethanol.[10] Because the ethanol molecule also has a nonpolar end, it will also dissolve nonpolar substances, including most essential oils[17] and numerous flavoring, coloring, and medicinal agents.
The addition of even a few percent of ethanol to water sharply reduces the surface tension of water. This property partially explains the “tears of wine” phenomenon. When wine is swirled in a glass, ethanol evaporates quickly from the thin film of wine on the wall of the glass. As the wine’s ethanol content decreases, its surface tension increases and the thin film “beads up” and runs down the glass in channels rather than as a smooth sheet.
Mixtures of ethanol and water that contain more than about 50% ethanol are flammable and easily ignited. An alcohol stove has been developed in India which runs on 50% ethanol/water mixture.[18] Alcoholic proof is a widely used measure of how much ethanol (i.e., alcohol) such a mixture contains. In the 18th century, proof was determined by adding a liquor (such as rum) to gunpowder. If the gunpowder still just exploded, that was considered to be “100 degrees proof” that it was “good” liquor — hence it was called “100 degrees proof.”
Ethanol-water solutions that contain less than 50% ethanol may also be flammable if the solution is first heated. Some cooking methods call for wine to be added to a hot pan, causing it to flash boil into a vapor, which is then ignited to burn off excess alcohol.
Ethanol is slightly more refractive than water, having a refractive index of 1.36242 (at λ=589.3 nm and 18.35 °C).[9]
[edit] Chemical properties
Ethanol is classified as a primary alcohol, meaning that the carbon to which its hydroxyl group is attached has at least two hydrogen atoms attached to it as well.
The chemistry of ethanol is largely that of its hydroxyl group.
[edit] Acid-base chemistry
Ethanol's hydroxyl group causes the molecule to be slightly basic. It is almost neutral like water. The pH of 100% ethanol is 7.33, compared to 7.00 for pure water. Ethanol can be quantitatively converted to its conjugate base, the ethoxide ion (CH3CH2O−), by reaction with an alkali metal such as sodium:[11]
or a very strong base such as sodium hydride:
- CH3CH2OH + NaH → CH3CH2ONa + H2.
This reaction is not possible in an aqueous solution, as water is more acidic, so that hydroxide is preferred over ethoxide formation.
[edit] Halogenation
Ethanol reacts with hydrogen halides to produce ethyl halides such as ethyl chloride and ethyl bromide:
HCl reaction requires a catalyst such as zinc chloride.[19] Hydrogen chloride in the presence of their respective zinc chloride is known as Lucas reagent.[11][19]
HBr requires refluxing with a sulfuric acid catalyst.[19]
Ethyl halides can also be produced by reacting ethanol with more specialized halogenating agents, such as thionyl chloride for preparing ethyl chloride, or phosphorus tribromide for preparing ethyl bromide.[11][19]
- CH3CH2OH + SOCl2 → CH3CH2Cl + SO2 + HCl
[edit] Haloform reaction
"The haloform reaction is a chemical reaction where a haloform (CHX3, where X is a halogen) is produced by the exhaustive halogenation of a methyl ketone (a molecule containing the R-CO-CH3 group) in the presence of a base.[20]" See main "Haloform reaction" article.
[edit] Ester formation
Under acid-catalyzed conditions, ethanol reacts with carboxylic acids to produce ethyl esters and water:
- RCOOH + HOCH2CH3 → RCOOCH2CH3 + H2O.
For this reaction to produce useful yields it is necessary to remove water from the reaction mixture as it is formed.
Ethanol can also form esters with inorganic acids. Diethyl sulfate and triethyl phosphate, prepared by reacting ethanol with sulfuric and phosphoric acid respectively, are both useful ethylating agents in organic synthesis. Ethyl nitrite, prepared from the reaction of ethanol with sodium nitrite and sulfuric acid, was formerly a widely-used diuretic.
[edit] Dehydration
Strong acid desiccants, such as sulfuric acid, cause ethanol's dehydration to form either diethyl ether or ethylene:
- 2 CH3CH2OH → CH3CH2OCH2CH3 + H2O
- CH3CH2OH → H2C=CH2 + H2O
Which product, diethyl ether or ethylene, predominates depends on the precise reaction conditions
[edit] Oxidation
Ethanol can be oxidized to acetaldehyde, and further oxidized to acetic acid. In the human body, these oxidation reactions are catalyzed by enzymes. In the laboratory, aqueous solutions of strong oxidizing agents, such as chromic acid or potassium permanganate, oxidize ethanol to acetic acid, and it is difficult to stop the reaction at acetaldehyde at high yield. Ethanol can be oxidized to acetaldehyde, without over oxidation to acetic acid, by reacting it with pyridinium chromic chloride.[19]
The direct oxidation of ethanol to acetic acid using chromic acid is given below.
- C2H5OH + 2[O] → CH3COOH + H2O
The oxidation product of ethanol, acetic acid, is spent as nutrient by the human body as acetyl CoA, where the acetyl group can be spent as energy or used for biosynthesis.
[edit] Chlorination
When exposed to chlorine, ethanol is both oxidized and its alpha carbon chlorinated to form the compound, chloral.
- 4Cl2 + C2H5OH → CCl3CHO + 5HCl
[edit] Combustion
Combustion of ethanol forms carbon dioxide and water:
- C2H5OH(g) + 3 O2(g) → 2 CO2(g) + 3 H2O(l); (ΔHr = −1409 kJ/mol[21])
[edit] Use
[edit] As a fuel
| Energy content of some fuels compared with ethanol:[22] | |||
|---|---|---|---|
| Fuel type | MJ/L | MJ/kg | Research octane number |
| Dry wood (20% moisture) | ~19.5 | ||
| Methanol | 17.9 | 19.9 | 123 |
| Ethanol | 23.5 | [23]31.1 | 129 |
| E85 (85% ethanol, 15% gasoline) |
25.2 | 33.2 | 105 |
| Liquefied natural gas | 25.3 | ~55 | |
| Autogas (LPG) (60% Propane + 40% Butane) |
26.8 | 50. | |
| Aviation gasoline (high-octane gasoline, not jet fuel) |
33.5 | 46.8 | |
| Gasohol (90% gasoline + 10% ethanol) |
33.7 | 47.1 | 93/94 |
| Regular Gasoline | 34.8 | [24]44.4 | min. 91 |
| Premium Gasoline | max. 95 | ||
| Diesel | 38.6 | 45.4 | 25 |
| Charcoal, extruded | 50 | 23 | |
The largest single use of ethanol is as a motor fuel and fuel additive. The largest national fuel ethanol industries exist in Brazil (gasoline sold in Brazil contains at least 25% ethanol and anhydrous ethanol is also used as fuel in more than 90% of new cars sold in the country). The Brazilian production of ethanol is praised for the high carbon sequestration capabilities of the sugar cane plantations, thus making it a real option to combat climate change.[25]
Henry Ford designed the first mass-produced automobile, the famed Model T Ford, to run on pure anhydrous (ethanol) alcohol—he said it was "the fuel of the future". Today, however, 100% pure ethanol is not approved as a motor vehicle fuel in the U.S. Added to gasoline, ethanol reduces ground-level ozone formation by lowering volatile organic compound and hydrocarbon emissions, decreasing carcinogenic benzene, and butadiene, emissions, and particulate matter emissions from gasoline combustion.[26]
Combustion of ethanol in an internal combustion engine yields many of the products of incomplete combustion that are produced by gasoline and significantly larger amounts of formaldehyde and related species such as formalin, acetaldehyde, etc..[27] This leads to a significantly larger photochemical reactivity that generates much more ground level ozone.[28] This data has been assembled into The Clean Fuels Report comparison of fuel emissions[29] and shows that ethanol exhaust generates 2.14 times as much ozone as does gasoline exhaust. When this is added into the custom "Localised Pollution Index (LPI)" of The Clean Fuels Report the local pollution, i.e. that which contributes to smog, is 1.7 on a scale where gasoline is 1.0 and higher numbers signify greater pollution. This issue has been formalised by the California Air Resources Board in 2008[citation needed] by recognising control standards for formaldehydes et al. as an emissions control group much like the conventional NOx and Reactive Organic Gases (ROGs).
Prior to the development of electronic fuel injection (EFI) and computerized engine management, the lower energy content of ethanol required that the engine carburetor be rejetted to permit a larger volume of fuel to mix with the intake air. EFI is able to actively compensate for varying fuel energy densities by monitoring the oxygen content of exhaust gases. However, a standard EFI gasoline engine can typically only tolerate up to 10% ethanol and 90% gasoline. Higher ethanol ratios require either larger-volume fuel injectors or an increase in fuel rail pressure to deliver the greater liquid volume needed to equal the energy content of pure gasoline.

World production of ethanol in 2006 was 51 gigalitres (1.3×1010 US gal), with 69% of the world supply coming from Brazil and the United States.[30] More than 20% of the Brazilian fleet of cars on the streets are able to use 100% ethanol as fuel, which includes ethanol-only engines and flex-fuel engines.[31] Flex-fuel engines in Brazil are able to work with all ethanol, all gasoline or any mixture of both. In the US flex-fuel vehicles can run on 0% to 85% ethanol (15% gasoline) since higher ethanol blends are not yet allowed. Brazil supports this population of ethanol-burning automobiles with large national infrastructure that produces ethanol from domestically grown sugar cane. Sugar cane not only has a greater concentration of sucrose than corn (by about 30%), but is also much easier to extract. The bagasse generated by the process is not wasted, but is utilized in power plants as a surprisingly efficient fuel to produce electricity.
The United States fuel ethanol industry is based largely on corn. According to the Renewable Fuels Association, as of October 30, 2007, 131 grain ethanol bio-refineries in the United States have the capacity to produce 7.0 billion US gallons (26 GL) of ethanol per year. An additional 72 construction projects underway (in the U.S.) can add 6.4 billion gallons of new capacity in the next 18 months. Over time, it is believed that a material portion of the ~150 billion gallon per year market for gasoline will begin to be replaced with fuel ethanol.[32]
The Energy Policy Act of 2005 requires that 4 billion gallons of "renewable fuel" be used in 2006 and this requirement will grow to a yearly production of 7.5 billion gallons by 2012.[33] In the United States, ethanol is most commonly blended with gasoline as a 10% ethanol blend nicknamed "gasohol". This blend is widely sold throughout the U.S. Midwest, and in cities required by the 1990 Clean Air Act to oxygenate their gasoline during the winter. Ethanol and isobutene are also the feedstocks for ethyl tert-butyl ether (ETBE), an oxygenate antiknock additive. The use of ethanol makes ETBE partially a biofuel, but also more expensive than the similar additive methyl tert-butyl ether (MTBE), made from methanol and isobutene.
Ethanol is also used as a cooking and lighting fuel. In India ethanol stove and lanterns have been developed which can run on 50% by weight ethanol/water mixture. This mixture (hooch or illicit liquor) is easy to distill, safer to handle and use than 100% ethanol, can be produced by small local producers and uses less energy in its production.[citation needed]
[edit] Food versus fuel debate

It is disputed whether corn ethanol as an automotive fuel results in a net energy gain or loss. As reported in "The Energy Balance of Corn Ethanol: an Update,"[34] the energy returned on energy invested (EROEI) for ethanol made from corn in the U.S. is 1.34 (it yields 34% more energy than it takes to produce it). Input energy includes natural gas based fertilizers, farm equipment, transformation from corn or other materials, and transportation. However, other researchers report that the production of ethanol consumes more energy than it yields.[35][36] In comparison, sugar cane ethanol EROEI is at around 8 (it yields 8 joules for each joule used to produce it).[citation needed] Recent research suggests that cellulosic crops such as switchgrass provide a much better net energy production than corn, producing over five times as much energy as the total used to produce the crop and convert it to fuel.[37] If this research is confirmed, cellulosic crops will most likely displace corn as the main fuel crop for producing bioethanol.
Michael Grunwald reports that one person could be fed for 1 year "on the corn needed to fill an ethanol-fueled SUV".[38] He further reports that though "hyped as an eco-friendly fuel, ethanol increases global warming, destroys forests and inflates food prices." Environmentalists, livestock farmers, and opponents of subsidies say that increased ethanol production won't meet energy goals and may damage the environment, while at the same time causing worldwide food prices to soar. Some of the controversial subsidies in the past have included more than $10 billion to Archer-Daniels-Midland since 1980.[39][neutrality disputed] Critics also speculate that as ethanol is more widely used, changing irrigation practices could greatly increase pressure on water resources. In October 2007, 28 environmental groups decried the Renewable Fuels Standard (RFS), a legislative effort intended to increase ethanol production, and said that the measure will "lead to substantial environmental damage and a system of biofuels production that will not benefit family farmers...will not promote sustainable agriculture and will not mitigate global climate change."[40][41]
Recent articles have also blamed subsidized ethanol production for the nearly 200% increase in milk prices since 2004[42], since the price of fuel has driven up the costs to cultivate, grow, harvest, ship, refine, bring to market, etc, all commodities including, but not limited to, milk, although that is disputed by some[citation needed]. Articles also blame the presence of speculators, and the recent growing interest in the commodities market by investors who have been scared away from a falling stock market[citation needed].
Ethanol production uses the starch portion of corn, but the leftover protein can be used to create a high-nutrient, low-cost animal feed.[43]
In 2007 the United Nations' independent expert on the right to food, called for a five-year moratorium on biofuel production from food crops, to allow time for development of non-food sources. He called recent increases in food costs because of fuel production, such as the quadrupling of world corn price in one year, a growing "catastrophe" for the poor.[44] In February 2007, riots occurred in Mexico because of the skyrocketing price of tortillas. Ethanol has been credited as the reason for this increase in food prices[45]. The demand for corn has had a rippling effect on many corn-based products, like tortillas. The effects of ethanol and the increasing cost of food have also been felt in Pakistan, Indonesia, and Egypt.[46]
Oil has historically had a much higher EROEI than corn produced ethanol, according to some[citation needed]. However, oil must be refined into gasoline before it can be used for automobile fuel. Refining, as well as exploration and drilling, consumes energy. The difference between the energy in the fuel (output energy) and the energy needed to produce it (input energy) is often expressed as a percent of the input energy and called net energy gain (or loss). Several studies released in 2002 estimated that the net energy gain for corn ethanol is between 21 and 34 percent. The net energy loss for MTBE is about 33 percent. When added to gasoline, ethanol can replace MTBE as an anti-knock agent without poisoning drinking water as MTBE does. In Brazil, where the broadest and longest ethanol producing experiment took place, improvements in agricultural practices and ethanol production improvements led to an increase in ethanol net energy gain from 300% to over 800% in recent years.[citation needed] It must be noted that Brazil produces ethanol more efficiently because its primary input is the sugar from sugar cane rather than starches from corn. Consuming known oil reserves is increasing oil exploration and drilling energy consumption which is reducing oil EROEI (and energy balance) further.[47]
Opponents claim that corn ethanol production does not result in a net energy gain or that the consequences of large scale ethanol production to the food industry and environment offset any potential gains from ethanol. It has been estimated that "if every bushel of U.S. corn, wheat, rice and soybean were used to produce ethanol, it would only cover about 4% of U.S. energy needs on a net basis."[48] Many of the issues raised could likely be fixed by techniques now in development that produce ethanol from agricultural waste, such as paper waste, switchgrass, and other materials, but EIA Forecasts Significant Shortfall in Cellulosic Biofuel Production Compared to Target Set by Renewable Fuel Standard.[49]
Proponents cite the potential gains to the U.S. economy both from domestic fuel production and increased demand for corn. Optimistic calculations project that the United States is capable of producing enough ethanol to completely replace gasoline consumption.[citation needed] In comparison, Brazil's ethanol consumption today covers more than 50% of all energy used by vehicles in that country.[50]
In the United States, preferential regulatory and tax treatment of ethanol automotive fuels introduces complexities beyond its energy economics alone. North American automakers have in 2006 and 2007 promoted a blend of 85% ethanol and 15% gasoline, marketed as E85, and their flex-fuel vehicles, e.g. GM's "Live Green, Go Yellow" campaign.[51] The apparent motivation is the nature of U.S. Corporate Average Fuel Economy (CAFE) standards, which give an effective 54% fuel efficiency bonus to vehicles capable of running on 85% alcohol blends over vehicles not adapted to run on 85% alcohol blends.[52] In addition to this auto manufacturer-driven impetus for 85% alcohol blends, the United States Environmental Protection Agency had authority to mandate that minimum proportions of oxygenates be added to automotive gasoline on regional and seasonal bases from 1992 until 2006 in an attempt to reduce air pollution, in particular ground-level ozone and smog.[53] In the United States, incidents of methyl tert(iary)-butyl ether (MTBE) groundwater contamination have been recorded in the majority of the 50 states,[54] and the State of California's ban on the use of MTBE as a gasoline additive has further driven the more widespread use of ethanol as the most common fuel oxygenate.[55]
A February 7, 2008 Associated Press article stated, "The widespread use of ethanol from corn could result in nearly twice the greenhouse gas emissions as the gasoline it would replace because of expected land-use changes, researchers concluded Thursday. The study challenges the rush to biofuels as a response to global warming."[56] The article does not take into account that even when grown in an industrial, soil-depleting manner, corn still sequesters carbon through its unharvested root and stalk tissues, which form soils, while gasoline production does not have a carbon-sequestration component in its production cycle.
One acre of land can yield about 7,110 pounds (3,225 kg) of corn, which can be processed into 328 gallons (1240.61 liters) of ethanol. That is about 26.1 pounds (11.84 kg) of corn per gallon.
Much overlooked in most discussions about ethanol from corn are the by-products from the production of ethanol. In general, the waste product from corn distillation is DDGS, distillers grains, a protein-rich food. The vast majority of corn produced in the US and the world goes to feed not people but livestock, which cannot naturally digest corn. The main result of feeding corn to a ruminant is excessive flatulence (production of methane gas, being a greenhouse gas), but the same animal can can readily digest DDGS[citation needed]. Seen in this light, all corn destined for livestock feeding should probably be distilled to harvest the ethanol fuel potential while simultaneously making the feed more nutritious to the livestock and avoiding unnecessary methane pollution. Wherever corn is used to feed livestock, farmers can take advantage of this process to make a profit on both food and fuel from the same bushel of corn.
However, there are supporters of the switch to ethanol fuel. A Federal Government Sponsored study found a gallon of ethanol makes almost twice as much energy as it consumes while it also has the potential to cut greenhouse gasses by 54% if cars ran on ethanol rather than gasoline.[57]
[edit] Ethanol fuel cells
Ethanol may be used as a fuel to power Direct-ethanol fuel cells (DEFC) in order to produce electricity and the by-products of water (H2O) and carbon dioxide (CO2).[58] Platinum is commonly used as an anode in such fuel cells in order to achieve a power density that is comparable to competing technologies. Until recently the high price of platinum has been cost prohibitive. A company called Acta Nanotech has created platinum free nanostructured anodes using more common and therefore less expensive metals.[59] A vehicle using a DEFC and non-platinum nanostructured anodes was used in the Shell Eco-Marathon 2007 by a team from Offenburg Germany which achieved an efficiency of 2716 kilometers per liter (6388 miles per gallon).[60]
[edit] Rocket fuel
Ethanol was commonly used as fuel in early bipropellant rocket vehicles, in conjunction with an oxidizer such as liquid oxygen. The German V-2 rocket of World War II, credited with beginning the space age, used ethanol, mixed with water to reduce the combustion chamber temperature.[61][62] The V-2's design team helped develop U.S. rockets following World War II, including the ethanol-fueled Redstone rocket, which launched the first U.S. satellite.[63] Alcohols fell into general disuse as more efficient rocket fuels were developed.[62]
[edit] Alcoholic beverages
Ethanol is the principal psychoactive constituent in alcoholic beverages, with depressant effects on the central nervous system. It has a complex mode of action and affects multiple systems in the brain, most notably ethanol acts as an agonist to the GABA receptors.[64] Similar psychoactives include those which also interact with GABA receptors, such as gamma-hydroxybutyric acid (GHB).[65] Ethanol is metabolized by the body as an energy-providing carbohydrate nutrient, as it metabolizes into acetyl CoA, an intermediate common with glucose metabolism, that can be used for energy in the citric acid cycle or for biosynthesis.
Alcoholic beverages vary considerably in their ethanol content and in the foodstuffs from which they are produced. Most alcoholic beverages can be broadly classified as fermented beverages, beverages made by the action of yeast on sugary foodstuffs, or as distilled beverages, beverages whose preparation involves concentrating the ethanol in fermented beverages by distillation. The ethanol content of a beverage is usually measured in terms of the volume fraction of ethanol in the beverage, expressed either as a percentage or in alcoholic proof units.
Fermented beverages can be broadly classified by the foodstuff from which they are fermented. Beers are made from cereal grains or other starchy materials, wines and ciders from fruit juices, and meads from honey. Cultures around the world have made fermented beverages from numerous other foodstuffs, and local and national names for various fermented beverages abound.
Distilled beverages are made by distilling fermented beverages. Broad categories of distilled beverages include whiskeys, distilled from fermented cereal grains; brandies, distilled from fermented fruit juices, and rum, distilled from fermented molasses or sugarcane juice. Vodka and similar neutral grain spirits can be distilled from any fermented material (grain or potatoes are most common); these spirits are so thoroughly distilled that no tastes from the particular starting material remain. Numerous other spirits and liqueurs are prepared by infusing flavors from fruits, herbs, and spices into distilled spirits. A traditional example is gin, which is created by infusing juniper berries into a neutral grain alcohol.
In a few beverages, ethanol is concentrated by means other than distillation. Applejack is traditionally made by freeze distillation, by which water is frozen out of fermented apple cider, leaving a more ethanol-rich liquid behind. Eisbier (more commonly, eisbock) is also freeze-distilled, with beer as the base beverage. Fortified wines are prepared by adding brandy or some other distilled spirit to partially-fermented wine. This kills the yeast and conserves some of the sugar in grape juice; such beverages are not only more ethanol-rich, but are often sweeter than other wines.
Alcoholic beverages are sometimes used in cooking, not only for their inherent flavors, but also because the alcohol dissolves hydrophobic flavor compounds which water cannot.
Just as industrial ethanol is used as feedstock for the production of industrial acetic acid, alcoholic beverages are made into culinary/household vinegar.
[edit] Feedstock
Ethanol is an important industrial ingredient and has widespread use as a base chemical for other organic compounds. These include ethyl halides, ethyl esters, diethyl ether, acetic acid, butadiene, and ethyl amines.
[edit] Antiseptic use
Ethanol is used in medical wipes and in most common antibacterial hand sanitizer gels at a concentration of about 62% (percentage by volume, not weight) as an antiseptic. Ethanol kills organisms by denaturing their proteins and dissolving their lipids and is effective against most bacteria and fungi, and many viruses (including SARS [2]), but is ineffective against bacterial spores.[66]
[edit] Antidote use
Ethanol can be used as an antidote for poisoning by other toxic alcohols, in particular methanol[67] and ethylene glycol. Ethanol competes with other alcohols for the alcohol dehydrogenase enzyme, preventing metabolism into toxic aldehyde and carboxylic acid derivatives.[68]
[edit] Other uses
- Ethanol is easily miscible in water and is a good solvent. Ethanol is less polar than water and is used in perfumes, paints and tinctures.
- Ethanol is also used in design and sketch art markers, such as Copic, and Tria.
- Alcohol is also found in certain kinds of deodorants.
[edit] Use in history
Before the development of modern medicines, ethanol was used for a variety of medical purposes. It has been known to be used as a truth drug (as hinted at by the maxim "in vino veritas"), as medicine for depression and as an anesthetic.
[edit] Effects on humans
[edit] Short-term
| BAC (mg/dL) | Symptoms[69] |
|---|---|
| 50 | Euphoria, talkativeness, relaxation |
| 100 | Central nervous system depression, impaired motor and sensory function, impaired cognition |
| >140 | Decreased blood flow to brain |
| 300 | Stupefaction, possible unconsciousness |
| 400 | Possible death |
| >550 | Death |
[edit] Effects on the central nervous system
Ethanol is a central nervous system depressant and has significant psychoactive effects in sublethal doses; for specifics, see effects of alcohol on the body by dose. Based on its abilities to change the human consciousness, ethanol is considered a drug.[70] Death from ethyl alcohol consumption is possible when blood alcohol level reaches 0.4%. A blood level of 0.5% or more is commonly fatal. Levels of even less than 0.1% can cause intoxication, with unconsciousness often occurring at 0.3–0.4%.[71]
The amount of ethanol in the body is typically quantified by blood alcohol content (BAC), the milligrams of ethanol per 100 milliliters of blood. The table at right summarizes the symptoms of ethanol consumption. Small doses of ethanol generally produce euphoria and relaxation; people experiencing these symptoms tend to become talkative and less inhibited, and may exhibit poor judgment. At higher dosages (BAC > 100 mg/dl), ethanol acts as a central nervous system depressant, producing at progressively higher dosages, impaired sensory and motor function, slowed cognition, stupefaction, unconsciousness, and possible death.
Prolonged heavy consumption of alcohol can cause significant permanent damage to the brain and other organs. See Alcohol consumption and health.
In America, about half of the deaths in car accidents occur in alcohol-related crashes.[72] There is no completely-safe level of alcohol for driving; the risk of a fatal car accident rises with the level of alcohol in the driver's blood.[73] However, most drunk driving laws governing the acceptable levels in the blood while driving or operating heavy machinery set typical upper limits of blood alcohol content (BAC) between 0.05% and 0.08%.
Discontinuing consumption of alcohol after several years of chronic drinking can also be fatal. Alcohol withdrawal can cause anxiety, autonomic dysfunction, seizures and hallucinations. Delirium tremens is a condition that requires people with a long history of heavy drinking to undertake an alcohol detoxification regimen.
[edit] Effects on metabolism
Ethanol within the human body is converted into acetaldehyde by alcohol dehydrogenase and then into acetic acid by acetaldehyde dehydrogenase. The product of the first step of this breakdown, acetaldehyde,[74] is more toxic than ethanol. Acetaldehyde is linked to most of the clinical effects of alcohol. It has been shown to increase the risk of developing cirrhosis of the liver,[65] multiple forms of cancer, and alcoholism.
[edit] Drug interactions
Ethanol can intensify the sedation caused by other central nervous system depressant drugs such as barbiturates, benzodiazepines, opioids, and phenothiazines[71]
[edit] Magnitude of effects
Some individuals have less-effective forms of one or both of the metabolizing enzymes, and can experience more-severe symptoms from ethanol consumption than others. Conversely, those who have acquired alcohol tolerance have a greater quantity of these enzymes, and metabolize ethanol more rapidly.[75]
[edit] Long-term
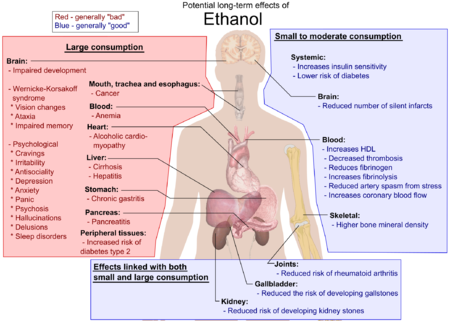
[edit] Birth defects
Ethanol is classified as a teratogen. See fetal alcohol syndrome.
[edit] Other effects
Frequent drinking of alcoholic beverages has been shown to be a major contributing factor in cases of elevated blood levels of triglycerides.[76]
Ethanol is not a carcinogen.[77][78] However, the first metabolic product of ethanol, acetaldehyde, is toxic, mutagenic, and carcinogenic.
[edit] Production
Ethanol is produced both as a petrochemical, through the hydration of ethylene, and biologically, by fermenting sugars with yeast.[79] Which process is more economical is dependent upon the prevailing prices of petroleum and of grain feed stocks.
[edit] Ethylene hydration
Ethanol for use as industrial feedstock is most often made from petrochemical feed stocks, typically by the acid-catalyzed hydration of ethylene, represented by the chemical equation
The catalyst is most commonly phosphoric acid,[80] adsorbed onto a porous support such as diatomaceous earth or charcoal. This catalyst was first used for large-scale ethanol production by the Shell Oil Company in 1947.[81] The reaction is carried out with an excess of high pressure steam at 300°C.
In an older process, first practiced on the industrial scale in 1930 by Union Carbide,[82] but now almost entirely obsolete, ethylene was hydrated indirectly by reacting it with concentrated sulfuric acid to produce ethyl sulfate, which was then hydrolyzed to yield ethanol and regenerate the sulfuric acid:[19]
- C2H4 + H2SO4 → CH3CH2SO4H
- CH3CH2SO4H + H2O → CH3CH2OH + H2SO4
[edit] Fermentation
Ethanol for use in alcoholic beverages, and the vast majority of ethanol for use as fuel, is produced by fermentation. When certain species of yeast (e.g., Saccharomyces cerevisiae) metabolize sugar they produce ethanol and carbon dioxide. The chemical equation below summarizes the conversion:
The process of culturing yeast under conditions to produce alcohol is called fermentation. Ethanol's toxicity to yeast limits the ethanol concentration obtainable by brewing. The most ethanol-tolerant strains of yeast can survive up to approximately 15% ethanol by volume.[83]
In order to produce ethanol from starchy materials such as cereal grains, the starch must first be converted into sugars. In brewing beer, this has traditionally been accomplished by allowing the grain to germinate, or malt, which produces the enzyme, amylase. When the malted grain is mashed, the amylase converts the remaining starches into sugars. For fuel ethanol, the hydrolysis of starch into glucose can be accomplished more rapidly by treatment with dilute sulfuric acid, fungally produced amylase, or some combination of the two.[84]
[edit] Cellulosic ethanol
Sugars for ethanol fermentation can be obtained from cellulose.[85][86] Until recently, however, the cost of the cellulase enzymes capable of hydrolyzing cellulose has been prohibitive. The Canadian firm Iogen brought the first cellulose-based ethanol plant on-stream in 2004.[87] Its primary consumer so far has been the Canadian government, which, along with the United States Department of Energy, has invested heavily in the commercialization of cellulosic ethanol. Deployment of this technology could turn a number of cellulose-containing agricultural by-products, such as corncobs, straw, and sawdust, into renewable energy resources. Other enzyme companies are developing genetically engineered fungi that produce large volumes of cellulase, xylanase, and hemicellulase enzymes. These would convert agricultural residues such as corn stover, wheat straw, and sugar cane bagasse and energy crops such as switchgrass into fermentable sugars.[88]
Cellulose-bearing materials typically also contain other polysaccharides, including hemicellulose. When hydrolyzed, hemicellulose decomposes into mostly five-carbon sugars such as xylose. S. cerevisiae, the yeast most commonly used for ethanol production, cannot metabolize xylose. Other yeasts and bacteria are under investigation to ferment xylose and other pentoses into ethanol.[89]
On January 14, 2008, General Motors announced a partnership with Coskata, Inc. The goal is to produce cellulosic ethanol cheaply, with an eventual goal of US$1 per U.S. gallon ($0.30/L) for the fuel. The partnership plans to begin producing the fuel in large quantity by the end of 2008. By 2011 a full-scale plant will come on line, capable of producing 50 to 100 million gallons of ethanol a year (200–400 ML/a).[90]
[edit] Prospective technologies
The anaerobic bacterium Clostridium ljungdahlii, recently discovered in commercial chicken wastes, can produce ethanol from single-carbon sources including synthesis gas, a mixture of carbon monoxide and hydrogen that can be generated from the partial combustion of either fossil fuels or biomass. Use of these bacteria to produce ethanol from synthesis gas has progressed to the pilot plant stage at the BRI Energy facility in Fayetteville, Arkansas.[91]
Another prospective technology is the closed-loop ethanol plant.[92] Ethanol produced from corn has a number of critics who suggest that it is primarily just recycled fossil fuels because of the energy required to grow the grain and convert it into ethanol. There is also the issue of competition with use of corn for food production. However, the closed-loop ethanol plant attempts to address this criticism. In a closed-loop plant, the energy for the distillation comes from fermented manure, produced from cattle that have been fed the by-products from the distillation. The leftover manure is then used to fertilize the soil used to grow the grain. Such a process is expected to lower the fossil fuel consumption used during conversion to ethanol by 75%.[93] Although energy can be created from the collection of methane from livestock manure, this can be mutually exclusive to the production of ethanol and should not be tagged on to it to make ethanol production seem more efficient or environmentally friendly.
Though in an early stage of research, there is some development of alternative production methods that use feed stocks such as municipal waste or recycled products, rice hulls, sugarcane bagasse, small diameter trees, wood chips, and switchgrass.[94]
[edit] Testing
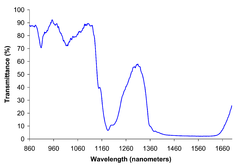
Breweries and biofuel plants employ two methods for measuring ethanol concentration. Infrared ethanol sensors measure the vibrational frequency of dissolved ethanol using the CH band at 2900 cm−1. This method uses a relatively inexpensive solid state sensor that compares the CH band with a reference band to calculate the ethanol content. The calculation makes use of the Beer-Lambert law. Alternatively, by measuring the density of the starting material and the density of the product, using a hydrometer, the change in specific gravity during fermentation indicates the alcohol content. This inexpensive and indirect method has a long history in the beer brewing industry.
[edit] Purification
Ethylene hydration or brewing produces an ethanol–water mixture. For most industrial and fuel uses, the ethanol must be purified. Fractional distillation can concentrate ethanol to 95.6% by volume (89.5 mole%). This mixture is an azeotrope with a boiling point of 78.1 °C, and cannot be further purified by distillation.
In one common industrial method to obtain absolute alcohol, a small quantity of benzene is added to rectified spirit and the mixture is then distilled. Absolute alcohol is obtained in the third fraction, which distills over at 78.3 °C (351.4 K).[11] Because a small amount of the benzene used remains in the solution, absolute alcohol produced by this method is not suitable for consumption, as benzene is carcinogenic.[95]
There is also an absolute alcohol production process by desiccation using glycerol. Alcohol produced by this method is known as spectroscopic alcohol—so called because the absence of benzene makes it suitable as a solvent in spectroscopy.
Other methods for obtaining absolute ethanol include desiccation using adsorbents such as starch or zeolites, which adsorb water preferentially, as well as azeotropic distillation and extractive distillation.
[edit] Grades of ethanol
[edit] Denatured alcohol
Pure ethanol and alcoholic beverages are heavily taxed, but ethanol has many uses that do not involve consumption by humans. To relieve the tax burden on these uses, most jurisdictions waive the tax when an agent has been added to the ethanol to render it unfit to drink. These include bittering agents such as denatonium benzoate and toxins such as methanol, naphtha, and pyridine. Products of this kind are called denatured alcohol.[96][97]
[edit] Absolute ethanol
Absolute or anhydrous alcohol generally refers to purified ethanol, containing no more than one percent water. Absolute alcohol not intended for human consumption often contains trace amounts of toxic benzene (used to remove water by azeotropic distillation). Consumption of this form of ethanol can be fatal over a short time period. Generally this kind of ethanol is used as solvents for lab and industrial settings where water will disrupt a desired reaction.
Pure ethanol is classed as 200 proof in the USA, equivalent to 175 degrees proof in the UK system.
[edit] See also
[edit] References
- ^ Myers, Richard L.; Myers, Rusty L. (2007). The 100 most important chemical compounds: a reference guide. Westport, Conn.: Greenwood Press. pp. 122. ISBN 0313337586.
- ^ Roach, J. (July 18, 2005). "9,000-Year-Old Beer Re-Created From Chinese Recipe". National Geographic News. http://news.nationalgeographic.com/news/2005/07/0718_050718_ancientbeer.html. Retrieved on 2007-09-03.
- ^ Hassan, Ahmad Y. "Alcohol and the Distillation of Wine in Arabic Sources". History of Science and Technology in Islam. http://www.history-science-technology.com/Notes/Notes%207.htm. Retrieved on 2008-03-29.
- ^ Alcohol in the Encyclopædia Britannica Eleventh Edition
- ^ Couper AS (1858). "On a new chemical theory" (online reprint). Philosophical magazine 16 (104–16). http://web.lemoyne.edu/~giunta/couper/couper.html. Retrieved on 2007-09-03.
- ^ Hennell, H. (1828). "On the mutual action of sulfuric acid and alcohol, and on the nature of the process by which ether is formed". Philosophical Transactions 118 (365–71): 365. doi:.
- ^ a b Robert Siegel (2007-02-15). "Ethanol, Once Bypassed, Now Surging Ahead". NPR. http://www.npr.org/templates/story/story.php?storyId=7426827. Retrieved on 2007-09-22.
- ^ Joseph DiPardo. "Outlook for Biomass Ethanol Production and Demand" (PDF). United States Department of Energy. http://tonto.eia.doe.gov/FTPROOT/features/biomass.pdf. Retrieved on 2007-09-22.
- ^ a b c CRC Handbook of Chemistry, 44th ed.
- ^ a b c d Windholz, Martha (1976). The Merck index: an encyclopedia of chemicals and drugs (9th ed.). Rahway, N.J., U.S.A: Merck. ISBN 0-911910-26-3.
- ^ a b c d e Morrison, Robert Thornton; Boyd, Robert Neilson (1972). Organic Chemistry (2nd ed.). Allyn and Bacon, inc..
- ^ Dahlmann U, Schneider GM (September 1989). "(Liquid + liquid) phase equilibria and critical curves of (ethanol + dodecane or tetradecane or hexadecane or 2,2,4,4,6,8,8-heptamethylnonane) from 0.1 MPa to 120.0 MPa". J Chem Thermodyn. 21 (9): 997–1004. doi:.
- ^ "Encyclopedia of chemical technology". Encyclopedia of chemical technology. 9. 1991. pp. 813.
- ^ Costigan MJ, Hodges LJ, Marsh KN, Stokes RH, Tuxford CW (1980). "The Isothermal Displacement Calorimeter: Design Modifications for Measuring Exothermic Enthalpies of Mixing". Aust J Chem. 33 (10): 2103–19. doi:. http://www.publish.csiro.au/nid/51/paper/CH9802103.htm.
- ^ Lei Z, Wang H, Zhou R, Duan Z (2002). "Influence of salt added to solvent on extractive distillation". Chem Eng J. 87: 149–56. doi:.
- ^ Pemberton RC, Mash CJ (September 1978). "Thermodynamic properties of aqueous non-electrolyte mixtures II. Vapour pressures and excess Gibbs energies for water + ethanol at 303.15 to 363.15 K determined by an accurate static method". J Chem Thermodyn. 10 (9): 867–88. doi:.
- ^ Merck Index of Chemicals and Drugs, 9th ed.; monographs 6575 through 6669
- ^ Anil K. Rajvanshi, S.M. Patil, and B. Mendonca (2007). "Low-concentration ethanol stove for rural areas in India". Energy for Sustainable Development 11 (1): 94–9. doi:.
- ^ a b c d e f Streitweiser, Andrew Jr.; Heathcock, Clayton H. (1976). Introduction to Organic Chemistry. MacMillan. ISBN 0-02-418010-6.
- ^ Chakrabartty, in Trahanovsky, Oxidation in Organic Chemistry, pp 343-370, Academic Press, New York, 1978
- ^ Frederick D. Rossini (1937). "Heats of Formation of Simple Organic Molecules". Ind. Eng. Chem. 29 (12): 1424–1430. doi:.
- ^ Appendix B, Transportation Energy Data Book from the Center for Transportation Analysis of the Oak Ridge National Laboratory
- ^ Calculated from heats of formation. Does not correspond exactly to the figure for MJ/l divided by density.
- ^ George. Overview of Storage Development DOE Hydrogen Program [pdf. Livermore, CA. Sandia National Laboratories. 2000.]
- ^ Reel, M. (August 19, 2006) "Brazil's Road to Energy Independence", Washington Post.
- ^ "Ethanol Fuel". http://journeytoforever.org/ethanol.html#E.
- ^ California Air Resources Board,Definition of a Low Emission Motor Vehicle in Compliance with the Mandates of Health and Safety Code Section 39037.05,second release, October 1989
- ^ A.Lowi& W.P.L.Carter; A Method for Evaluating the Atmospheric Ozone Impact of Actual Vehicle emissions, S.A.E. Technical Paper, Warrendale,PA; march 1990
- ^ T.T.M.Jones,The Clean Fuels Report: A Quantitative Comparison Of Motor Fuels, Related Pollution and Technologies: 2008.[1]
- ^ "Renewable Fuels Association Industry Statistics". http://www.ethanolrfa.org/industry/statistics/#E.
- ^ "Tecnologia flex atrai estrangeiros". Agência Estado. http://www.estadao.com.br/economia/not_eco178105,0.htm.
- ^ "First Commercial U.S. Cellulosic Ethanol Biorefinery Announced". Renewable Fuels Association. 2006-11-20. http://www.ethanolrfa.org/media/press/rfa/view.php?id=909. Retrieved on May 21 2006.
- ^ "Renewable Fuel Standard Program". United States Environmental Protection Agency. 2007-04-10. http://www.epa.gov/otaq/renewablefuels/. Retrieved on May 21 2007.
- ^ Hosein Shapouri, James A. Duffield, and Michael Wang. "The Energy Balance of Corn Ethanol: an Update" (PDF). United States Department of Agriculture. http://www.transportation.anl.gov/pdfs/AF/265.pdf. Retrieved on May 21 2007.
- ^ Pimentel D, Patzek TW (2005). "Ethanol Production Using Corn, Switchgrass, and Wood; Biodiesel Production Using Soybean and Sunflower". Natural Resources Research 14 (1): 65–76. doi:.
- ^ Lang, Susan S.. "Cornell ecologist's study finds that producing ethanol and biodiesel from corn and other crops is not worth the energy". Cornell University. http://www.news.cornell.edu/stories/July05/ethanol.toocostly.ssl.html. Retrieved on July 5 2005.
- ^ M.R. Schmer, K.P. Vogel, R.B. Mitchell, R.K. Perrin (2007-11-21). "Net energy of cellulosic ethanol from switchgrass". U.S. Dept. of Agrigulture. http://www.pnas.org/cgi/reprint/0704767105v1. Retrieved on 2008-01-13.
- ^ The Clean Energy Scam, TIME, April 7, 2008, pages 40–41.
- ^ Doug Bandow (1997-10-02). "Ethanol Keeps ADM Drunk On Tax Dollars.". CATO Institute. http://www.cato.org/pub_display.php?pub_id=6079. Retrieved on 2007-09-03.
- ^ The Politics of Ethanol Outshine its Costs
- ^ Moira Herbst (March 19, 2007). "Ethanol's Growing List of Enemies". Business Week. http://www.businessweek.com/bwdaily/dnflash/content/mar2007/db20070316_016207.htm?campaign_id=rss_topStories. Retrieved on 2007-09-03.
- ^ Jeff Cox (June 19, 2007). "Corn and milk: A 1-2 inflation combo". CNNMoney.com. http://money.cnn.com/2007/06/19/news/economy/commodity_prices/index.htm. Retrieved on 2007-09-03.
- ^ Fuel, Food Demand Raise Corn, Soybean Prices
- ^ Edith M. Lederer, Associated Press (2007-10-27). "UN Expert Calls Biofuel 'Crime Against Humanity'". http://www.livescience.com/environment/071027-ap-biofuel-crime.html.
- ^ Mexicans stage tortilla protest , BBC News, February 7, 2007.
- ^ Posted by Hans Bader (2008-04-08). "Ethanol Subsidies Cause Food Riots in Mexico, Pakistan, Indonesia, Yemen, and Egypt". Open Market blog. http://www.openmarket.org/2008/04/08/ethanol-subsidies-cause-food-riots-in-mexico-pakistan-indonesia-yemen-and-egypt.
- ^ David Andress & Associates (November 2002). "Ethanol Energy Balances". http://oregon.gov/ENERGY/RENEW/Biomass/forum.shtml.
- ^ "Forget the Ethanol Myth -- Avoid Biofuel Bubble: John F. Wasik". Bloomberg.com. 2007-07-23. http://www.bloomberg.com/apps/news?pid=20601039&refer=columnist_wasik&sid=aOS8e5kvDESE. Retrieved on 2007-07-25.
- ^ "Study Finds Net Energy of Cellulosic Ethanol from Switchgrass Much Higher Than Expected". Green Car Congress. 2008-01-07. http://www.greencarcongress.com/cellulosic_ethanol/index.html. Retrieved on 2008-01-13.
- ^ Agência Brasil (2008-07-15). "ANP: consumo de álcool combustível é 50% maior em 2007" (in Portuguese). Invertia. http://br.invertia.com/noticias/noticia.aspx?idNoticia=200807152306_ABR_77211977. Retrieved on 2008-08-09.
- ^ "GM Announces E85 Fuel Card Promotion On FlexFuel Vehicles". The Auto Channel. http://theautochannel.com/news/2006/05/02/005502.html. Retrieved on 2007-09-04.
- ^ "CAFE Credits for Flex Fuel Vehicles Undermine Improvements in Fuel Economy". Public Citizen. 2006-09-27. http://www.citizen.org/autosafety/vehicles/enviro/articles.cfm?ID=15763. Retrieved on 2007-09-03.
- ^ "Regulations & Standards". United States Environmental Protection Agency. http://www.epa.gov/otaq/rfg_regs.htm. Retrieved on 2007-09-04.
- ^ "MTBE Contamination from Underground Storage Tanks" (PDF). United States General Accounting Office. 2002-05-21. http://gao.gov/new.items/d02753t.pdf. Retrieved on 2007-10-09.
- ^ "Methyl Tertiary Butyl Ether (MTBE)". United State Environmental Protection Agency. http://www.epa.gov/mtbe/faq.htm. Retrieved on 2007-09-04.
- ^ Study: Ethanol May Add to Global Warming Associated Press, February 7, 2008
- ^ "Ethanol—Better Than We Thought?". PopSci.com.au. 2009-01-30. http://www.popsci.com.au/environment/article/2009-01/ethanol%E2%80%94better-we-thought. Retrieved on 2009-01-30.
- ^ Direct Methanol Fuel Cells (DMFC) FCTec.
- ^ Offenburg students test world's first ethanol powered fuel cell vehicle Acta.
- ^ Willkommen beim Projekt "Schluckspecht" der Hochschule Offenburg .
- ^ David Darling. "The Internet Encyclopedia of Science: V-2". http://daviddarling.info/encyclopedia/V/V-2.html.
- ^ a b Braeunig, Robert A. "Rocket Propellants." (Website). Rocket & Space Technology, 2006. Retrieved on 2007-08-23.
- ^ "A Brief History of Rocketry." NASA Historical Archive, via science.ksc.nasa.gov.
- ^ Chastain G (2006). "Alcohol, neurotransmitter systems, and behavior". The Journal of general psychology 133 (4): 329–35. doi:. PMID 17128954.
- ^ a b Dr. Bill Boggan. "Effects of Ethyl Alcohol on Organ Function". Chemases.com. http://chemcases.com/alcohol/alc-07.htm. Retrieved on 2007-09-29.
- ^ McDonnell G, Russell AD (January 1, 1999). "Antiseptics and disinfectants: activity, action, and resistance". Clin. Microbiol. Rev. 12 (1): 147–79. PMID 9880479. PMC: 88911. http://cmr.asm.org/cgi/pmidlookup?view=long&pmid=9880479.
- ^ "Methanol Poisoning". Cambridge University School of Clinical Medicine. http://www-clinpharm.medschl.cam.ac.uk/pages/teaching/topics/poison/poison9.html. Retrieved on 2007-09-04.
- ^ Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA (2002). "American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning". J. Toxicol. Clin. Toxicol. 40 (4): 415–46. doi:. PMID 12216995.
- ^ Pohorecky LA, Brick J (1988). "Pharmacology of ethanol". Pharmacol. Ther. 36 (2-3): 335–427. doi:. PMID 3279433. http://linkinghub.elsevier.com/retrieve/pii/0163-7258(88)90109-X.
- ^ MedlinePlus Encyclopedia Alcohol Use
- ^ a b David A. Yost, MD (December 2002) ([dead link] – Scholar search). Acute care for alcohol intoxication. 112. Postgraduate Medicine Online. http://postgradmed.com/issues/2002/12_02/yost1.htm. Retrieved on 2007-09-29.
- ^ Hingson R, Winter M (2003). "Epidemiology and consequences of drinking and driving" (PDF). Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism 27 (1): 63–78. PMID 15301401. http://pubs.niaaa.nih.gov/publications/arh27-1/63-78.pdf.
- ^ Naranjo CA, Bremner KE (1993). "Behavioural correlates of alcohol intoxication". Addiction 88 (1): 25–35. doi:. PMID 8448514.
- ^ Dr. Bill Boggan. "Metabolism of Ethyl Alcohol in the Body". Chemases.com. http://chemcases.com/alcohol/alc-06.htm. Retrieved on 2007-09-29.
- ^ Agarwal DP, Goedde HW (1992). "Pharmacogenetics of alcohol metabolism and alcoholism". Pharmacogenetics 2 (2): 48–62. doi:. PMID 1302043.
- ^ "Triglycerides". American Heart Association. http://americanheart.org/presenter.jhtml?identifier=4778. Retrieved on 2007-09-04.
- ^ "Material Data Safety Sheet" (PDF). Burdick and Jackson. http://www.cise.columbia.edu/clean/msds/ethanol.pdf. Retrieved on 2007-10-25.
- ^ Pollyanna R. Chavez, Xiang-Dong Wang, Jean Meyer. "Animal Models for Carcinogenesis and Chemoprevention, abstract #C42: Effects of Chronic Ethanol Intake on Cyclin D1 Levels and Altered Foci in Diethylnitrosamine-initiated Rats" (PDF). USDA. http://www.aacr.org/PDF_files/2004Prevention/Program/2004_Prevention_Abstracts.pdf. Retrieved on 2007-10-24.
- ^ Mills, G.A.; Ecklund, E.E. "Mills GA, Ecklund EE (1987). "Alcohols as Components of Transportation Fuels". Annual Review of Energy 12: 47–80. doi:. http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.eg.12.110187.000403?journalCode=energy.
- ^ Roberts, John D.; Caserio, Marjorie C. (1977). Basic Principles of Organic Chemistry. W. A. Benjamin, Inc. ISBN 0-8053-8329-8.
- ^ "Encyclopedia of chemical technology". Encyclopedia of chemical technology. 9. 1991. pp. 82-.
- ^ Lodgsdon, J.E. (1994). p. 817
- ^ Morais PB, Rosa CA, Linardi VR, Carazza F, Nonato EA (November 1996). "Production of fuel alcohol by Saccharomyces strains from tropical habitats". Biotechnology Letters 18 (11): 1351–6. doi:. http://www.springerlink.com/content/h32k825756g41318/.
- ^ Badger, P.C. "Ethanol From Cellulose: A General Review." p. 17–21. In: J. Janick and A. Whipkey (eds.), Trends in new crops and new uses. ASHS Press, 2002, Alexandria, VA. Retrieved on September 2, 2007.
- ^ Taherzadeh MJ, Karimi K (2007). "Acid-based hydrolysis processes for ethanol from lignocellulosic materials: A review". BioResources 2 (3): 472–99.
- ^ Taherzadeh MJ, Karimi K (2007). "Enzymatic-based hydrolysis processes for ethanol from lignocellulosic materials: A review". BioResources 2 (4): 707–38.
- ^ Ritter SK (May 31, 2004). "Biomass or Bust". Chemical & Engineering News 82 (22): 31–4.
- ^ Clines, Tom (July 2006). "Brew Better Ethanol". Popular Science Online. http://www.popsci.com/popsci/energy/6756226d360ab010vgnvcm1000004eecbccdrcrd.html.
- ^ Dhinakar S. Kompala. "Maximizing Ethanol Production by Engineered Pentose-Fermenting Zymononas mobilis" (PDF). Department of Chemical Engineering, University of Colorado at Boulder. http://www.metabolicengineering.gov/me2001/2001Kompala.pdf. Retrieved on May 21 2007.
- ^ Mick, Jason (2008-01-14). "Cellulosic Ethanol Promises $1 per Gallon Fuel From Waste". DailyTech.com. http://www.dailytech.com/Cellulosic+Ethanol+Promises+1+per+Gallon+Fuel+From+Waste/article10320.htm. Retrieved on 2008-01-15.
- ^ "Providing for a Sustainable Energy Future". Bioengineering Resources, inc. http://www.brienergy.com/. Retrieved on May 21 2007.
- ^ Closed-Loop Ethanol Plant to Start Production. www.renewableenergyaccess.com. November 2, 2006. http://www.renewableenergyaccess.com/rea/news/story?id=46414. Retrieved on 2007-09-03.
- ^ Rapier, R. (June 26, 2006) "E3 Biofuels: Responsible Ethanol" R-Squared Energy Blog
- ^ "Air Pollution Rules Relaxed for US Ethanol Producers". Truthout. 2007-04-12. http://www.truthout.org/issues_06/041607ED.shtml. Retrieved on May 21 2007.
- ^ Snyder R, Kalf GF (1994). "A perspective on benzene leukemogenesis". Crit. Rev. Toxicol. 24 (3): 177–209. doi:. PMID 7945890.
- ^ "U-M Program to Reduce the Consumption of Tax-free Alcohol; Denatured Alcohol a Safer, Less Expensive Alternative" (PDF). University of Michigan. http://www.procurement.umich.edu/Contracts/Denatured_Alchohol.pdf. Retrieved on 2007-09-29.
- ^ Great Britain (2005). The Denatured Alcohol Regulations 2005. Statutory Instrument 2005 No. 1524.
[edit] Further reading
- The National Institute on Alcohol Abuse and Alcoholism maintains a database of alcohol-related health effects. [1]
- "Alcohol." (1911). In Hugh Chisholm (Ed.) Encyclopædia Britannica Eleventh Edition. Online reprint
- Lodgsdon, J.E (1991). "Encyclopedia of chemical technology". in Howe-Grant, Mary; Kirk, Raymond E.; Othmer, Donald F.; Kroschwitz, Jacqueline I.. Encyclopedia of chemical technology. 9 (4th ed.). New York: Wiley. pp. 812–60. ISBN 0-471-52669-X.
- Smith, M.G., and M. Snyder. (2005). "Ethanol-induced virulence of Acinetobacter baumannii". American Society for Microbiology meeting. June 5 – June 9. Atlanta.
- Sci-toys website explanation of US denatured alcohol designations
- Boyce, John M., and Pittet Didier. (2003). “Hand Hygiene in Healthcare Settings.” Centers for Disease Control, Atlanta, Georgia, United States.
- Rene Martinez VitalSensors Technologies LLC. "VS1000A Series In-Line Ethanol Sensors for the Beverage and BioFuel Industry" (PDF). http://vitalsensorstech.com/VitalSensors%20VS-1000A%20Ethanol%20Alcohol%20Sensor%20Data%20Sheet.pdf. Martinez describes the theory and practice of measuring brix on-line in beverages.
[edit] External links
- International Labour Organization ethanol safety information
- National Pollutant Inventory – Ethanol Fact Sheet
- National Institute of Standards and Technology chemical data on ethanol
- ChEBI – biology related
- Chicago Board of Trade news and market data on ethanol futures
- Calculation of vapor pressure, liquid density, dynamic liquid viscosity, surface tension of ethanol
- Biofuels News
|
||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
- ^ "ETOH Archival Database (1972–2003)". Alcohol and Alcohol Problems Science Database. NIAAA. http://etoh.niaaa.nih.gov/Archive.htm. Retrieved on 2008-03-31.