IUPAC nomenclature of organic chemistry
From Wikipedia, the free encyclopedia
The IUPAC nomenclature of organic chemistry is a systematic method of naming organic chemical compounds as recommended[1] by the International Union of Pure and Applied Chemistry (IUPAC). Ideally, every organic compound should have a name from which an unambiguous structural formula can be drawn. There is also an IUPAC nomenclature of inorganic chemistry. See also phanes nomenclature of highly complex cyclic molecules.
The main idea of IUPAC nomenclature is that every compound has one and only one name, and every name corresponds to only one structure of molecules (i.e. a one-one relationship), thereby reducing ambiguity.
For ordinary communication, to spare a tedious description, the official IUPAC naming recommendations are not always followed in practice except when it is necessary to give a concise definition to a compound, or when the IUPAC name is simpler (viz. ethanol against ethyl alcohol). Otherwise the common or trivial name may be used, often derived from the source of the compound (See Sec 14. below)
Contents |
[edit] Basic principles
| This article contains instructions, advice, or how-to content. The purpose of Wikipedia is to present facts, not to teach subject matter. Please help improve this article either by rewriting the how-to content or by moving it to Wikiversity or Wikibooks. |
In chemistry, a number of prefixes, suffixes and infixes are used to describe the type and position of functional groups in the compound.
The steps to naming an organic compound are:
- Identify the parent hydrocarbon chain (The longest continuous chain of carbon atoms)
- Identify the functional group, if any (If more than one, use the one with highest precedence as shown here)
- Identify the position of the functional group in the chain.
- Number the carbon atoms in the parent chain. The functional group should end up the least number possible (as there are two ways of numbering—right to left and left to right). The number (in Arabic numerals, i.e. 1, 2, 3....) is written before the name of the functional group suffix (such as -ol, -one, -al, etc.). If the group is a group that can only exist at the end of any given chain (such as the carboxylic acid and aldehyde groups), it need not be numbered.
NOTE: If there are no functional groups, number in both directions, find the numbers of the side-chains (the carbon chains that are not in the parent chain) in both directions. The end result should be such that the first number should be the least possible. In the event of the first numbers being the same for two methods of numbering, the sum of the numbers of the side chains should be made the least possible; for example, 2,2,5-trimethylhexane (2 + 2 + 5 = 9) is preferred over 2,5,5-trimethylhexane (2 + 5 + 5 = 12), as they both start with '2', but the sum of the numbers of the former is more.
- Identify the side-chains and number them. Side chains are the carbon chains that are not in the parent chain, but are branched off from it.
If there is more than one of the same type of side-chain, add the prefix (di-, tri-, etc.) before it. The numbers for that type of side chain will be grouped in ascending order and written before the name of the side-chain. If there are two side-chains with the same alpha carbon, the number will be written twice. Example: 2,2,3-trimethyl... - Identify the remaining functional groups, if any, and name them by the name of their ions (Such as hydroxy for -OH, oxy for =O , oxyalkane for O-R, etc.).
Different side-chains and functional groups will be grouped together in alphabetical order. (The prefixes di-, tri-, etc. are not taken into consideration for grouping alphabetically. For example, ethyl comes before dihydroxy or dimethyl, as the "e" in "ethyl" precedes the "h" in "hydroxy" and the "m" in "dimethyl" alphabetically. The "di" is not considered in both cases.) In the case of there being both side chains and secondary functional groups, they should be written mixed together in one group rather than in two separate groups. - Identify double/triple bonds. Number them with the number of the carbon atom at the head of the bond (i.e the carbon atom with the lesser number that it is attached to). For example a double bond between carbon atoms 3 and 4 is numbered as 3-ene. Multiple bonds of one type (double/triple) are named with a prefix (di-, tri-, etc.). If both types of bonds exist, then use "ene" before "yne" e.g. "6 13 diene 19 yne"If all bonds are single, use "ane" without any numbers or prefixes.
- Arrange everything like this: Group of side chains and secondary functional groups with numbers made in step 3 + prefix of parent hydrocarbon chain (eth, meth) + double/triple bonds with numbers (or "ane") + primary functional group suffix with numbers.
Wherever it says "with numbers", it is understood that between the word and the numbers, you use the prefix(di-, tri-) - Add punctuation:
- Put commas between numbers (2 5 5 becomes 2,5,5)
- Put a hyphen between a number and a letter (2 5 5 trimethylhexane becomes 2,5,5-trimethylhexane)
- Successive words are merged into one word (trimethyl hexane becomes trimethylhexane)
NOTE: IUPAC uses one-word names throughout. This is why all parts are connected.
The finalized name should look like this:
#,#-di<side chain>-#-<secondary functional group><-#-<side chain>-#,#,#-tri<secondary functional group><parent chain suffix><If all bonds are single bonds, use "ane">-#,#-di<double bonds>-#-<triple bonds>-#-<primary functional group>
NOTE: # is used for a number. The group secondary functional groups and side chains may not look the same as shown here, as the side chains and secondary functional groups are arranged alphabetically. The di- and tri- have been used just to show their usage. (di- after #,#, tri- after #,#,# , etc.)
Example:
Here is a sample molecule with the parent carbons numbered:
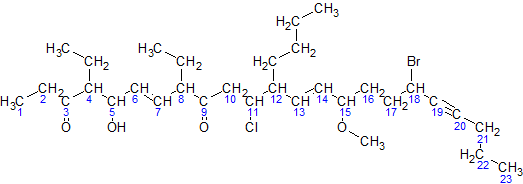
For simplicity, here is an image of the same molecule, where the hydrogens in the parent chain are removed and the carbons are shown by their numbers:

Now, we go by the steps:
- The parent hydrocarbon chain has 23 carbons. It is called tricos-.
- The functional groups with the highest precedence are the two ketone groups.
- The groups are on carbon atoms 3 & 9. As there are two, we write 3,9-dione.
- The numbering of the molecule is based on the ketone groups. when numbering from left to right, the ketone groups get numbered 3 and 9.when numbering from right to left, the ketone groups get numbered 15 and 21. The sum of 3 & 9 (12) is less than the sum of 15 & 21 (36). Therefore, the numbering s done left to right, and the ketones are numbered 3 & 9.
- The side chains are: an ethyl- at carbon 4, an ethyl- at carbon 8, and a butyl- at carbon 12.
NOTE:The -O-CH3 at carbon atom 15 is not a side chain, but it is a methoxy functional group- There are two ethyl- groups, so they are combined to create, 4,8-diethyl.
- The side chains shall be grouped like this: 12-butyl-4,8-diethyl. (But this is not the final grouping, as functional groups may be added in between.)
- The secondary functional groups are: a hydroxy- at carbon 5, a chloro- at carbon 11, a methoxy- at carbon 15, and a bromo- at carbon 18. Grouped with the side chains, we get 18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxy
- There are two double bonds: one between carbons 6 & 7, and one between carbons 13 & 14. They will be called 6,13-diene. There is one triple bond between carbon atoms 19 & 20. It will be called 19-yne
- The arrangement(with punctuation) is: 18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxytricos-6,13-diene-19-yne-3,9-dione
The final name is 18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxytricos-6,13-diene-19-yne-3,9-dione.
[edit] Alkanes
Straight-chain alkanes take the suffix "-ane" and are prefixed depending on the number of carbon atoms in the chain, following standard rules. The first few are:
| Number of carbons | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 20 | 30 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prefix | Meth | Eth | Prop | But | Pent | Hex | Hept | Oct | Non | Dec | Undec | Dodec | Tridec | Tetradec | Pentadec | Eicos | Triacont |
For example, the simplest alkane is CH4 methane, and the nine-carbon alkane CH3(CH2)7CH3 is named nonane. The names of the first four alkanes were derived from methanol, ether, propionic acid and butyric acid, respectively. The rest are named with a Greek numeric prefix, with the exceptions of nonane which has a Latin prefix, and undecane and tridecane which have mixed-language prefixes.
Cyclic alkanes are simply prefixed with "cyclo-", for example C4H8 is cyclobutane and C6H12 is cyclohexane.
Branched alkanes are named as a straight-chain alkane with attached alkyl groups. They are prefixed with a number indicating the carbon the group is attached to, counting from the end of the alkane chain. For example, (CH3)2CHCH3, commonly known as isobutane, is treated as a propane chain with a methyl group bonded to the middle (2) carbon, and given the systematic name 2-methylpropane. However, although the name 2-methylpropane COULD be used, it is easier and more logical to call it simply methylpropane - the methyl group could not possible occur on any of the other cabon atoms (that would lengthen the chain and result in butane, not propane) and therefore the use of the number "2" is not necessary.
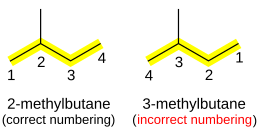
If there is ambiguity in the position of the substituent, depending on which end of the alkane chain is counted as "1", then numbering is chosen so that the smallest number is used. For example, (CH3)2CHCH2CH3 (isopentane) is named 2-methylbutane, not 3-methylbutane.
If there are multiple side-branches of the same size alkyl group, their positions are separated by commas and the group prefixed with di-, tri-, tetra-, etc., depending on the number of branches (e.g. C(CH3)4 2,2-dimethylpropane). If there are different groups, they are added in alphabetical order, separated by commas or hyphens: 3-ethyl-4-methylhexane. The longest possible main alkane chain is used; therefore 3-ethyl-4-methylhexane instead of 2,3-diethylpentane, even though these describe equivalent structures. The di-, tri- etc. prefixes are ignored for the purpose of alphabetical ordering of side chains (e.g. 3-ethyl-2,4-dimethylpentane, not 2,4-dimethyl-3-ethylpentane).
[edit] Alkenes and Alkynes
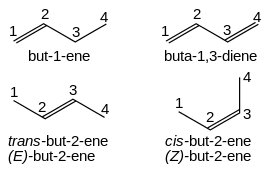
Alkenes are named for their parent alkane chain with the suffix "-ene" and an infixed number indicating the position of the double-bonded carbon in the chain: CH2=CHCH2CH3 is but-1-ene. Multiple double bonds take the form -diene, -triene, etc., with the size prefix of the chain taking an extra "a": CH2=CHCH=CH2 is buta-1,3-diene. Simple cis and trans isomers are indicated with a prefixed cis- or trans-: cis-but-2-ene, trans-but-2-ene. More complex geometric isomerisations are described using the Cahn Ingold Prelog priority rules.
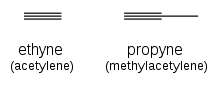
Alkynes are named using the same system, with the suffix "-yne" indicating a triple bond: ethyne (acetylene), propyne (methylacetylene).
[edit] Functional Groups
[edit] Table of Functional groups
Alk is the prefix of the group (Meth, Eth, Prop, etc.)
[edit] Alcohols
Alcohols (R-OH) take the suffix "-ol" with an infix numerical bonding position: CH3CH2CH2OH is propan-1-ol. The suffixes -diol, -triol, -tetraol, etc., are used for multiple -OH groups: Ethylene glycol CH2OHCH2OH is ethane-1,2-diol.
If higher precedence functional groups are present (see order of precedence, below), the prefix "hydroxy" is used with the bonding position: CH3CHOHCOOH is 2-hydroxypropanoic acid.
[edit] Halogens (Alkyl Halides)
Halogen functional groups are prefixed with the bonding position and take the form fluoro-, chloro-, bromo-, iodo-, etc., depending on the halogen. Multiple groups are dichloro-, trichloro-, etc, and dissimilar groups are ordered alphabetically as before. For example, CHCl3 (chloroform) is trichloromethane. The anesthetic Halothane (CF3CHBrCl) is 2-bromo-2-chloro-1,1,1-trifluoroethane.
[edit] Ketones
In general ketones (R-CO-R) take the suffix "-one" (pronounced own, not won) with an infix position number: CH3CH2CH2COCH3 is pentan-2-one. if a higher precedence suffix is in use, the prefix "oxo-" is used: CH3CH2CH2COCH2CHO is 3-oxohexanal.
[edit] Aldehydes
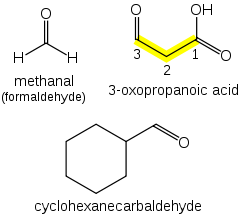
Aldehydes (R-CHO) take the suffix "-al".If other functional groups are present, the chain is numbered such that the aldehyde carbon is in the "1" position.
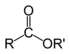
If a prefix form is required, "oxo-" is used (as for ketones), with the position number indicating the end of a chain: CHOCH2COOH is 3-oxopropanoic acid. If the carbon in the carbonyl group cannot be included in the attached chain (for instance in the case of cyclic aldehydes), the prefix "formyl-" or the suffix "-carbaldehyde" is used: C6H11CHO is cyclohexanecarbaldehyde. If a aldehyde is attached to a benzene and is the main functional group, the suffix becomes benzaldehyde.
[edit] Carboxylic acids
In general carboxylic acids are named with the suffix -oic acid (etymologically a back-formation from benzoic acid). As for aldehydes, they take the "1" position on the parent chain, but do not have their position number indicated. For example, CH3CH2CH2CH2COOH (valeric acid) is named pentanoic acid. For common carboxylic acids some traditional names such as acetic acid are in such widespread use they are considered retained IUPAC names, although "systematic" names such as ethanoic acid are also acceptable. For carboxylic acids attached to a benzene ring such as Ph-COOH, these are named as benzoic acid or its derivatives.
If there are multiple carboxyl groups on the same parent chain, the suffix "-carboxylic acid" can be used (as -dicarboxylic acid, -tricarboxylic acid, etc.). In these cases, the carbon in the carboxyl group does not count as being part of the main alkane chain. The same is true for the prefix form, "carboxyl-". Citric acid is one example; it is named 2-hydroxypropane- 1,2,3-tricarboxylic acid, rather than 2-carboxy, 2-hydroxypentanedioic acid.
[edit] Ethers
Ethers (R-O-R) consist of an oxygen atom between the two attached carbon chains. The shorter of the two chains becomes the first part of the name with the -ane suffix changed to -oxy, and the longer alkane chain become the suffix of the name of the ether. Thus CH3OCH3 is methoxymethane, and CH3OCH2CH3 is methoxyethane (not ethoxymethane). If the oxygen is not attached to the end of the main alkane chain, then the whole shorter alkyl-plus-ether group is treated as a side-chain and prefixed with its bonding position on the main chain. Thus CH3OCH(CH3)2 is 2-methoxypropane.
[edit] Esters
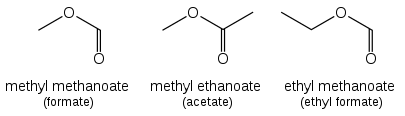
Esters (R-CO-O-R') are named as alkyl derivatives of carboxylic acids. The alkyl (R') group is named first. The R-CO-O part is then named as a separate word based on the carboxylic acid name, with the ending changed from -oic acid to -oate. For example, CH3CH2CH2CH2COOCH3 is methyl pentanoate, and (CH3)2CHCH2CH2COOCH2CH3 is ethyl 4-methylpentanoate. For esters such as ethyl acetate (CH3COOCH2CH3), ethyl formate (HCOOCH2CH3) or dimethyl phthalate that are based on common acids, IUPAC recommends use of these established names, called retained names. The -oate changes to -ate. Some simple examples, named both ways, are shown in the figure above.
If the alkyl group is not attached at the end of the chain, the bond position to the ester group is infixed before "-yl": CH3CH2CH(CH3)OOCCH2CH3 may be called but-2-yl propanoate or but-2-yl propionate.
[edit] Amines and Amides
Amines (R-NH2) are named for the attached alkane chain with the suffix "-amine" (e.g. CH3NH2 methanamine). If necessary, the bonding position is infixed: CH3CH2CH2NH2 propan-1-amine, CH3CHNH2CH3 propan-2-amine. The prefix form is "amino-".
For secondary amines (of the form R-NH-R), the longest carbon chain attached to the nitrogen atom becomes the primary name of the amine; the other chain is prefixed as an alkyl group with location prefix given as an italic N: CH3NHCH2CH3 is N-methylethanamine. Tertiary amines (R-NR-R) are treated similarly: CH3CH2N(CH3)CH2CH2CH3 is N-ethyl-N-methylpropanamine. Again, the substituent groups are ordered alphabetically.
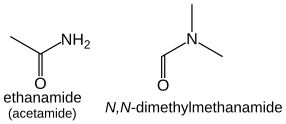
Amides (R-CO-NH2) take the suffix "-amide". There is no prefix form, and no location number is required since they always terminate a carbon chain, e.g. CH3CONH2 (acetamide) is named ethanamide.
Secondary and tertiary amides are treated similarly to the case of amines: alkane chains bonded to the nitrogen atom are treated as substituents with the location prefix N: HCON(CH3)2 is N,N-dimethylmethanamide.
[edit] Cyclic compounds
Cycloalkanes and aromatic compounds can be treated as the main parent chain of the compound, in which case the position of substituents are numbered around the ring structure. For example, the three isomers of xylene CH3C6H4CH3, commonly the ortho-, meta-, and para- forms, are 1,2-dimethylbenzene, 1,3-dimethylbenzene, and 1,4-dimethylbenzene. The cyclic structures can also be treated as functional groups themselves, in which case they take the prefix "cycloalkyl-" (e.g. "cyclohexyl-") or for benzene, "phenyl-".
The IUPAC nomenclature scheme becomes rapidly more elaborate for more complex cyclic structures, with notation for compounds containing conjoined rings, and many common names such as phenol, furan, indole, etc. being accepted as base names for compounds derived from them.
[edit] Order of precedence of groups
When compounds contain more than one functional group, the order of precedence determines which groups are named with prefix or suffix forms. The highest precedence group takes the suffix, with all others taking the prefix form. However, double and triple bonds only take suffix form (-en and -yn) and are used with other suffixes.
Prefixed substituents are ordered alphabetically (excluding any modifiers such as di-, tri-, etc.), e.g. chlorofluoromethane, not fluorochloromethane. If there are multiple functional groups of the same type, either prefixed or suffixed, the position numbers are ordered numerically (thus ethane-1,2-diol, not ethane-2,1-diol.) The N position indicator for amines and amides comes before "1", e.g. CH3CH(CH3)CH2NH(CH3) is N,2-dimethylpropanamine.
| Priority | Functional group | Formula | Prefix | Suffix |
|---|---|---|---|---|
| 1 | Cations e.g. Ammonium |
–NH4+ |
-onio- ammonio- |
-onium -ammonium |
| 2 | Carboxylic acids Thiocarboxylic acids Selenocarboxylic acids Sulfonic acids Sulfinic acids Sulfenic acids |
–COOH –COSH –COSeH –SO3H –SO2H –SOH |
carboxy- thiocarboxy- selenocarboxy- sulfo- sulfino- sulfeno- |
-oic acid* -thioic acid* -selenoic acid* -sulfonic acid -sulfinic acid -sulfenic acid |
| 3 | Carboxylic acid derivatives Esters Acyl halides Amides Imides Amidines |
–COOR –COX –CONH2 –CON=C< –C(=NH)NH2 |
R-oxycarbonyl- halidealcanoyl- carbamoyl- -imido- amidino- |
-R-oate -oyl halide* -amide* -imide* -amidine* |
| 4 | Nitriles Isocyanides |
–CN –NC |
cyano- isocyano- |
-nitrile* isocyanide |
| 5 | Aldehydes Thioaldehydes |
–CHO –CHS |
formyl- thioformyl- |
-al* -thial* |
| 6 | Ketones Thioketones |
>CO >CS |
oxo- thiono- |
-one -thione |
| 7 | Alcohols Thiols Selenols Tellurols |
–OH –SH –SeH –TeH |
hydroxy- sulfanyl- selanyl- tellanyl- |
-ol -thiol -selenol -tellurol |
| 8 | Hydroperoxides | –OOH | hydroperoxy- | -hydroperoxide |
| 9 | Amines Imines Hydrazines |
–NH2 =NH –NHNH2 |
amino- imino- hydrazino- |
-amine -imine -hydrazine |
| 10 | Ethers Thioethers Selenoethers |
–O– –S– –Se– |
-oxy- -thio- -seleno- |
|
| 11 | Peroxides Disulfides |
–OO– –SS– |
-peroxy- -disulfanyl- |
*Note: These suffixes, in which the carbon atom is counted as part of the preceding chain, are the most commonly used. See individual functional group articles for more details.
[edit] Common nomenclature - trivial names
Common nomenclature is an older system of naming organic compounds. Instead of using the prefixes for the carbon skeleton above, another system is used. The pattern can be seen below.
| Number of carbons | Prefix as in new system | Common name for alcohol | Common name for aldehyde | Common name for acid |
|---|---|---|---|---|
| 1 | Meth | Methyl alcohol (wood alcohol) | Formaldehyde | Formic acid |
| 2 | Eth | Ethyl alcohol (grain alcohol) | Acetaldehyde | Acetic acid |
| 3 | Prop | Propyl alcohol | Propionaldehyde | Propionic acid |
| 4 | But | Butyl alcohol | Butyraldehyde | Butyric acid |
| 5 | Pent | Amyl alcohol | Valeraldehyde | Valeric acid |
| 6 | Hex | - | Caproaldehyde | Caproic acid |
| 7 | Hept | Enanthyl alcohol | Enanthaldehyde | Enanthoic acid |
| 8 | Oct | Capryl alcohol | Caprylaldehyde | Caprylic acid |
| 9 | Non | - | Pelargonaldehyde | Pelargonic acid |
| 10 | Dec | Capric alcohol | Capraldehyde | Capric acid |
| 11 | Undec | - | - | - |
| 12 | Dodec | Lauryl alcohol | Lauraldehyde | Lauric acid |
| 13 | Tridec | - | - | - |
| 14 | Tetradec | - | Myristaldehyde | Myristic acid |
| 15 | Pentadec | - | - | - |
| 16 | Hexadec | Cetyl alcohol | Palmitaldehyde | Palmitic acid |
| 17 | Heptadec | - | - | Margaric acid |
| 18 | Octadec | Stearyl alcohol | Stearaldehyde | Stearic acid |
| 19 | Nonadec | - | - | - |
| 20 | Eicos | Arachidyl alcohol | - | Arachidic acid |
| 21 | Heneicos | - | - | - |
| 22 | Docos | Behenyl alcohol | - | Behenic acid |
| 23 | Tricos | - | - | - |
| 24 | Tetracos | Lignoceryl alcohol | - | Lignoceric acid |
| 26 | Hexacos | Cerotinyl alcohol | - | Cerotinic acid |
| 28 | Octacos | Montanyl alcohol | - | Montanic acid |
| 30 | Triacont | Melissyl alcohol | - | Melissic acid |
[edit] Ketones
Common names for ketones can be derived by naming the two alkyl or aryl groups bonded to the carbonyl group as separate words followed by the word ketone.
The first three of the names shown above are still considered to be acceptable IUPAC names.
[edit] Aldehydes
The common name for an aldehyde is derived from the common name of the corresponding carboxylic acid by dropping the word acid and changing the suffix from -ic or -oic to -aldehyde.
[edit] Ions
The IUPAC nomenclature also provides rules for naming ions.
[edit] Hydron
Hydron is a generic term for hydrogen cation; protons, deuterons and tritons are all hydrons.
[edit] Parent hydride cations
Simple cations formed by adding a hydron to a hydride of a halogen, chalcogen or nitrogen-family element are named by adding the suffix "-onium" to the element's root: H4N+ is ammonium, H3O+ is oxonium, and H2F+ is fluoronium. Ammonium was adopted instead of nitronium, which commonly refers to NO2+.
If the cationic center of the hydride is not a halogen, chalcogen or nitrogen-family element then the suffix "-ium" is added to the name of the neutral hydride after dropping any final 'e'. H5C+ is methanium, HO-O+H2 is dioxidanium (HO-OH is dioxidane), and H2N-N+H3 is diazanium (H2N-NH2 is diazane).
[edit] Cations and substitution
The above cations except for methanium are not, strictly speaking, organic, since they do not contain carbon. However, many organic cations are obtained by substituting another element or some functional group for a hydrogen.
The name of each substitution is prefixed to the hydride cation name. If many substitutions by the same functional group occur, then the number is indicated by prefixing with "di-", "tri-" as with halogenation. (CH3)3O+ is trimethyloxonium. CH3F3N+ is trifluoromethylammonium.
[edit] See also
[edit] References
- ^ (in English)Nomenclature of Organic Chemistry (3 ed.). London: Butterworths. 1971 (3rd edition combined). ISBN 0408701447.
- ^ Nomenclature of Organic Chemistry, Oxford: Pergamon Press, 1979; A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Oxford: Blackwell Scientific Publications, 1993.
[edit] External links
- IUPAC Nomenclature of Organic Chemistry (online version of the "Blue Book")
- IUPAC Recommendations on Organic & Biochemical Nomenclature, Symbols, Terminology, etc. (includes IUBMB Recommendations for biochemistry)
- Bibliography of IUPAC Recommendations on Organic Nomenclature (last updated 2003-04-11)
- ACD/Name Software for generating systematic nomenclature
- G. A. Eller, Improving the Quality of Published Chemical Names with Nomenclature Software. Molecules 2006, 9, 915-928 |(online article)
|
|||||