Light-emitting diode
From Wikipedia, the free encyclopedia
| Light-emitting diode | |
 Red, green and blue LEDs of the 5mm type |
|
| Type | Passive, optoelectronic |
|---|---|
| Working principle | Electroluminescence |
| Invented | Nick Holonyak Jr. (1962) |
| Electronic symbol | |
| Pin configuration | Anode and Cathode |
A light-emitting diode (LED) (pronounced /ˌɛliːˈdiː/[1], or just /lɛd/), is an electronic light source. The LED was first invented in Russia in the 1920s, and introduced in America as a practical electronic component in 1962. Oleg Vladimirovich Losev was a radio technician who noticed that diodes used in radio receivers emitted light when current was passed through them. In 1927, he published details in a Russian journal of the first ever LED.
All early devices emitted low-intensity red light, but modern LEDs are available across the visible, ultraviolet and infra red wavelengths, with very high brightness.
LEDs are based on the semiconductor diode. When the diode is forward biased (switched on), electrons are able to recombine with holes and energy is released in the form of light. This effect is called electroluminescence and the color of the light is determined by the energy gap of the semiconductor. The LED is usually small in area (less than 1 mm2) with integrated optical components to shape its radiation pattern and assist in reflection.[2]
LEDs present many advantages over traditional light sources including lower energy consumption, longer lifetime, improved robustness, smaller size and faster switching. However, they are relatively expensive and require more precise current and heat management than traditional light sources.
Applications of LEDs are diverse. They are used as low-energy and also for replacements for traditional light sources in well-established applications such as indicators and automotive lighting. The compact size of LEDs has allowed new text and video displays and sensors to be developed, while their high switching rates are useful in communications technology.
Contents |
[edit] History
[edit] Discoveries and early devices
Electroluminescence was discovered in 1907 by the British experimenter H. J. Round of Marconi Labs, using a crystal of silicon carbide and a cat's-whisker detector.[3] Russian Oleg Vladimirovich Losev independently created the first LED in the mid 1920s; his research was distributed in Russian, German and British scientific journals,[4][5] but no practical use was made of the discovery for several decades. Rubin Braunstein of the Radio Corporation of America reported on infrared emission from gallium arsenide (GaAs) and other semiconductor alloys in 1955.[6] Braunstein observed infrared emission generated by simple diode structures using gallium antimonide (GaSb), GaAs, indium phosphide (InP), and silicon-germanium (SiGe) alloys at room temperature and at 77 kelvin.
In 1961, experimenters Bob Biard and Gary Pittman working at Texas Instruments,[7] found that GaAs emitted infrared radiation when electric current was applied and received the patent for the infrared LED.
The first practical visible-spectrum (red) LED was developed in 1962 by Nick Holonyak Jr., while working at General Electric Company.[8] Holonyak is seen as the "father of the light-emitting diode".[9] M. George Craford[10], a former graduate student of Holonyak, invented the first yellow LED and improved the brightness of red and red-orange LEDs by a factor of ten in 1972.[11] In 1976, T.P. Pearsall created the first high-brightness, high efficiency LEDs for optical fiber telecommunications by inventing new semiconductor materials specifically adapted to optical fiber transmission wavelengths.[12]
Up to 1968 visible and infrared LEDs were extremely costly, on the order of US $200 per unit, and so had little practical application.[13] The Monsanto Corporation was the first organization to mass-produce visible LEDs, using gallium arsenide phosphide in 1968 to produce red LEDs suitable for indicators.[13] Hewlett Packard (HP) introduced LEDs in 1968, initially using GaAsP supplied by Monsanto. The technology proved to have major applications for alphanumeric displays and was integrated into HP's early handheld calculators.
[edit] Practical use
| This section needs additional citations for verification. Please help improve this article by adding reliable references (ideally, using inline citations). Unsourced material may be challenged and removed. (March 2009) |

The first commercial LEDs were commonly used as replacements for incandescent indicators, and in seven-segment displays,[14] first in expensive equipment such as laboratory and electronics test equipment, then later in such appliances as TVs, radios, telephones, calculators, and even watches (see list of signal applications). These red LEDs were bright enough only for use as indicators, as the light output was not enough to illuminate an area. Later, other colors became widely available and also appeared in appliances and equipment. As the LED materials technology became more advanced, the light output was increased, while maintaining the efficiency and the reliability to an acceptable level. The invention and development of the high power white light LED led to use for illumination[15] [16] (see list of illumination applications). Most LEDs were made in the very common 5 mm T1¾ and 3 mm T1 packages, but with increasing power output, it has become increasingly necessary to shed excess heat in order to maintain reliability[17], so more complex packages have been adapted for efficient heat dissipation. Packages for state-of-the-art high power LEDs bear little resemblance to early LEDs.
[edit] Continuing development

The first high-brightness blue LED was demonstrated by Shuji Nakamura of Nichia Corporation and was based on InGaN borrowing on critical developments in GaN nucleation on sapphire substrates and the demonstration of p-type doping of GaN which were developed by Isamu Akasaki and H. Amano in Nagoya. In 1995, Alberto Barbieri at the Cardiff University Laboratory (GB) investigated the efficiency and reliability of high-brightness LEDs demonstrated a very impressive result by using a transparent contact made of indium tin oxide (ITO) on (AlGaInP/GaAs) LED. The existence of blue LEDs and high efficiency LEDs quickly led to the development of the first white LED, which employed a Y3Al5O12:Ce, or "YAG", phosphor coating to mix yellow (down-converted) light with blue to produce light that appears white. Nakamura was awarded the 2006 Millennium Technology Prize for his invention.[18]
The development of LED technology has caused their efficiency and light output to increase exponentially, with a doubling occurring about every 36 months since the 1960s, in a way similar to Moore's law. The advances are generally attributed to the parallel development of other semiconductor technologies and advances in optics and material science. This trend is normally called Haitz's Law after Dr. Roland Haitz. [19]
In February 2008, Bilkent university in Turkey reported 300 lumens of visible light per watt luminous efficacy (not per electrical watt) and warm light by using nanocrystals [20].
[edit] Technology
[edit] Physics
Like a normal diode, the LED consists of a chip of semiconducting material impregnated, or doped, with impurities to create a p-n junction. As in other diodes, current flows easily from the p-side, or anode, to the n-side, or cathode, but not in the reverse direction. Charge-carriers—electrons and holes—flow into the junction from electrodes with different voltages. When an electron meets a hole, it falls into a lower energy level, and releases energy in the form of a photon.
The wavelength of the light emitted, and therefore its color, depends on the band gap energy of the materials forming the p-n junction. In silicon or germanium diodes, the electrons and holes recombine by a non-radiative transition which produces no optical emission, because these are indirect band gap materials. The materials used for the LED have a direct band gap with energies corresponding to near-infrared, visible or near-ultraviolet light.
LED development began with infrared and red devices made with gallium arsenide. Advances in materials science have made possible the production of devices with ever-shorter wavelengths, producing light in a variety of colors.
LEDs are usually built on an n-type substrate, with an electrode attached to the p-type layer deposited on its surface. P-type substrates, while less common, occur as well. Many commercial LEDs, especially GaN/InGaN, also use sapphire substrate.
Most materials used for LED production have very high refractive indices. This means that much light will be reflected back in to the material at the material/air surface interface. Therefore Light extraction in LEDs is an important aspect of LED production, subject to much research and development.
[edit] Efficiency and operational parameters
Typical indicator LEDs are designed to operate with no more than 30–60 milliwatts [mW] of electrical power. Around 1999, Philips Lumileds introduced power LEDs capable of continuous use at one watt [W]. These LEDs used much larger semiconductor die sizes to handle the large power inputs. Also, the semiconductor dies were mounted onto metal slugs to allow for heat removal from the LED die.
One of the key advantages of LED-based lighting is its high efficiency, as measured by its light output per unit power input. White LEDs quickly matched and overtook the efficiency of standard incandescent lighting systems. In 2002, Lumileds made five-watt LEDs available with a luminous efficiency of 18–22 lumens per watt [lm/W]. For comparison, a conventional 60–100 W incandescent lightbulb produces around 15 lm/W, and standard fluorescent lights produce up to 100 lm/W. A recurring problem is that efficiency will fall dramatically for increased current. This effect is know as droop and effectively limits the light output of a given LED, increasing heating more than light output for increased current.
In September 2003, a new type of blue LED was demonstrated by the company Cree, Inc. to provide 24 mW at 20 milliamperes [mA]. This produced a commercially packaged white light giving 65 lm/W at 20 mA, becoming the brightest white LED commercially available at the time, and more than four times as efficient as standard incandescents. In 2006 they demonstrated a prototype with a record white LED luminous efficiency of 131 lm/W at 20 mA. Also, Seoul Semiconductor has plans for 135 lm/W by 2007 and 145 lm/W by 2008, which would be approaching an order of magnitude improvement over standard incandescents and better even than standard fluorescents.[21] Nichia Corporation has developed a white light LED with luminous efficiency of 150 lm/W at a forward current of 20 mA.[22]
It should be noted that high-power (≥ 1 W) LEDs are necessary for practical general lighting applications. Typical operating currents for these devices begin at 350 mA. The highest efficiency high-power white LED is claimed[23] by Philips Lumileds Lighting Co. with a luminous efficiency of 115 lm/W (350 mA).
Cree issued a press release on November 19, 2008 about a laboratory prototype LED achieving 161 lumens/watt at room temperature. The total output was 173 lumens, and the correlated color temperature was reported to be 4689 K.[24][unreliable source?]
[edit] Lifetime and failure
Solid state devices such as LEDs are subject to very limited wear and tear if operated at low currents and at low temperatures. Many of the LEDs produced in the 1970s and 1980s are still in service today. Typical lifetimes quoted are 25000 to 100000 hours but heat and current settings can extend or shorten this time significantly. [25]
The most common symptom of LED (and diode laser) failure is the gradual lowering of light output and loss of efficiency. Sudden failures, although rare, can occur as well. Early red LEDs were notable for their short lifetime. With the development of high power LEDs the devices are subjected to higher junction temperatures and higher current densities than traditional devices. This causes stress on the material and may cause early light output degradation. To quantitatively classify lifetime in a standardized manner it has been suggested to use the terms L75 and L50 which is the time it will take a given LED to reach 75% and 50% light output respectively.[26]
[edit] Colors and materials
Conventional LEDs are made from a variety of inorganic semiconductor materials, the following table shows the available colors with wavelength range, voltage drop and material:
| Color | Wavelength [nm] | Voltage [V] | Semiconductor Material | |
|---|---|---|---|---|
| Infrared | λ > 760 | ΔV < 1.9 | Gallium arsenide (GaAs) Aluminium gallium arsenide (AlGaAs) |
|
| Red | 610 < λ < 760 | 1.63 < ΔV < 2.03 | Aluminium gallium arsenide (AlGaAs) Gallium arsenide phosphide (GaAsP) Aluminium gallium indium phosphide (AlGaInP) Gallium(III) phosphide (GaP) |
|
| Orange | 590 < λ < 610 | 2.03 < ΔV < 2.10 | Gallium arsenide phosphide (GaAsP) Aluminium gallium indium phosphide (AlGaInP) Gallium(III) phosphide (GaP) |
|
| Yellow | 570 < λ < 590 | 2.10 < ΔV < 2.18 | Gallium arsenide phosphide (GaAsP) Aluminium gallium indium phosphide (AlGaInP) Gallium(III) phosphide (GaP) |
|
| Green | 500 < λ < 570 | 2.18 < ΔV < 4.0 | Indium gallium nitride (InGaN) / Gallium(III) nitride (GaN) Gallium(III) phosphide (GaP) Aluminium gallium indium phosphide (AlGaInP) Aluminium gallium phosphide (AlGaP) |
|
| Blue | 450 < λ < 500 | 2.48 < ΔV < 3.7 | Zinc selenide (ZnSe) Indium gallium nitride (InGaN) Silicon carbide (SiC) as substrate Silicon (Si) as substrate — (under development) |
|
| Violet | 400 < λ < 450 | 2.76 < ΔV < 4.0 | Indium gallium nitride (InGaN) | |
| Purple | multiple types | 2.48 < ΔV < 3.7 | Dual blue/red LEDs, blue with red phosphor, or white with purple plastic |
|
| Ultraviolet | λ < 400 | 3.1 < ΔV < 4.4 | diamond (C) Aluminium nitride (AlN) Aluminium gallium nitride (AlGaN) Aluminium gallium indium nitride (AlGaInN) — (down to 210 nm[27]) |
|
| White | Broad spectrum | ΔV = 3.5 | Blue/UV diode with yellow phosphor |
[edit] Ultraviolet and blue LEDs

Blue LEDs are based on the wide band gap semiconductors GaN (gallium nitride) and InGaN (indium gallium nitride). They can be added to existing red and green LEDs to produce the impression of white light, though white LEDs today rarely use this principle.
The first blue LEDs were made in 1971 by Jacques Pankove (inventor of the gallium nitride LED) at RCA Laboratories.[28] However, these devices had too little light output to be of much practical use. In the late 1980s, key breakthroughs in GaN epitaxial growth and p-type doping by Isamu Akasaki and Hiroshi Amano (Nagoya, Japan)[29] ushered in the modern era of GaN-based optoelectronic devices. Building upon this foundation, in 1993 high brightness blue LEDs were demonstrated through the work of Shuji Nakamura at Nichia Corporation.[30]
By the late 1990s, blue LEDs had become widely available. They have an active region consisting of one or more InGaN quantum wells sandwiched between thicker layers of GaN, called cladding layers. By varying the relative InN-GaN fraction in the InGaN quantum wells, the light emission can be varied from violet to amber. AlGaN aluminium gallium nitride of varying AlN fraction can be used to manufacture the cladding and quantum well layers for ultraviolet LEDs, but these devices have not yet reached the level of efficiency and technological maturity of the InGaN-GaN blue/green devices. If the active quantum well layers are GaN, as opposed to alloyed InGaN or AlGaN, the device will emit near-ultraviolet light with wavelengths around 350–370 nm. Green LEDs manufactured from the InGaN-GaN system are far more efficient and brighter than green LEDs produced with non-nitride material systems.
With nitrides containing aluminium, most often AlGaN and AlGaInN, even shorter wavelengths are achievable. Ultraviolet LEDs in a range of wavelengths are becoming available on the market. Near-UV emitters at wavelengths around 375–395 nm are already cheap and often encountered, for example, as black light lamp replacements for inspection of anti-counterfeiting UV watermarks in some documents and paper currencies. Shorter wavelength diodes, while substantially more expensive, are commercially available for wavelengths down to 247 nm.[31] As the photosensitivity of microorganisms approximately matches the absorption spectrum of DNA, with a peak at about 260 nm, UV LEDs emitting at 250–270 nm are to be expected in prospective disinfection and sterilization devices. Recent research has shown that commercially available UVA LEDs (365 nm) are already effective disinfection and sterilization devices.[32]
Wavelengths down to 210 nm were obtained in laboratories using aluminium nitride.
While not an LED as such, an ordinary NPN bipolar transistor will emit violet light if its emitter-base junction is subjected to non-destructive reverse breakdown. This is easy to demonstrate by filing the top off a metal-can transistor (BC107, 2N2222 or similar) and biasing it well above emitter-base breakdown (≥ 20 V) via a current-limiting resistor.
[edit] White light
There are two ways of producing high intensity white-light using LEDs. One is to use individual LEDs that emit three primary colors[33] – red, green, and blue, and then mix all the colors to produce white light. The other is to use a phosphor material to convert monochromatic light from a blue or UV LED to broad-spectrum white light, much in the same way a fluorescent light bulb works.
[edit] RGB Systems

White light can be produced by mixing differently colored light, the most common method is to use red, green and blue (RGB). Hence the method is called multi-colored white LEDs (sometimes referred to as RGB LEDs). Because its mechanism is involved with sophisticated electro-optical design to control the blending and diffusion of different colors, this approach has rarely been used to mass produce white LEDs in the industry. Nevertheless this method is particularly interesting to many researchers and scientists because of the flexibility of mixing different colors.[34] In principle, this mechanism also has higher quantum efficiency in producing white light.
There are several types of multi-colored white LEDs: di-, tri-, and tetrachromatic white LEDs. Several key factors that play among these different approaches include color stability, color rendering capability, and luminous efficiency. Often higher efficiency will mean lower color rendering, presenting a trade off between the luminous efficiency and color rendering. For example, the dichromatic white LEDs have the best luminous efficiency (120 lm/W), but the lowest color rendering capability. Oppositely although tetrachromatic white LEDs have excellent color rendering capability, they often have poor luminous efficiency. Trichromatic white LEDs are in between, having both good luminous efficiency (>70 lm/W) and fair color rendering capability.
What multi-color LEDs offer is not merely another solution of producing white light, but is a whole new technique of producing light of different colors. In principle, most perceivable colors can be produced by mixing different amounts of three primary colors, and this makes it possible to produce precise dynamic color control as well. As more effort is devoted to investigating this technique, multi-color LEDs should have profound influence on the fundamental method which we use to produce and control light color. However, before this type of LED can truly play a role on the market, several technical problems need to be solved. These certainly include that this type of LED's emission power decays exponentially with increasing temperature,[35] resulting in a substantial change in color stability. Such problem is not acceptable for industrial usage. Therefore, many new package designs aiming to solve this problem have been proposed, and their results are being reproduced by researchers and scientists.
[edit] Phosphor based LEDs

This method involves coating an LED of one color (mostly blue LED made of InGaN) with phosphor of different colors to produce white light, the resultant LEDs are called phosphor based white LEDs. A fraction of the blue light undergoes the Stokes shift being transformed from shorter wavelengths to longer. Depending on the color of the original LED, phosphors of different colors can be employed. If several phosphor layers of distinct colors are applied, the emitted spectrum is broadened, effectively increasing the color rendering index (CRI) value of a given LED.
Phosphor based LEDs have a lower efficiency than normal LEDs due to the heat loss from the Stokes shift and also other phosphor-related degradation issues. However, the phosphor method is still the most popular technique for manufacturing high intensity white LEDs. The design and production of a light source or light fixture using a monochrome emitter with phosphor conversion is simpler and cheaper than a complex RGB system, and the majority of high intensity white LEDs presently on the market are manufactured using phosphor light conversion.
The greatest barrier to high efficiency is the seemingly unavoidable Stokes energy loss. However, much effort is being spent on optimizing these devices to higher light output and higher operation temperatures. For instance, the efficiency can be increased by adapting better package design or by using a more suitable type of phosphor. Philips Lumileds' patented conformal coating process addresses the issue of varying phosphor thickness, giving the white LEDs a more homogeneous white light. With development ongoing, the efficiency of phosphor based LEDs is generally increased with every new product announcement.
Technically the phosphor based white LEDs encapsulate InGaN blue LEDs inside of a phosphor coated epoxy. A common yellow phosphor material is cerium-doped yttrium aluminium garnet (Ce3+:YAG).
White LEDs can also be made by coating near ultraviolet (NUV) emitting LEDs with a mixture of high efficiency europium-based red and blue emitting phosphors plus green emitting copper and aluminium doped zinc sulfide (ZnS:Cu, Al). This is a method analogous to the way fluorescent lamps work. This method is less efficient than the blue LED with YAG:Ce phosphor, as the Stokes shift is larger and more energy is therefore converted to heat, but yields light with better spectral characteristics, which render color better. Due to the higher radiative output of the ultraviolet LEDs than of the blue ones, both approaches offer comparable brightness. Another concern is that UV light may leak from a malfunctioning light source and cause harm to human eyes or skin.
[edit] Other white LEDs
Another method used to produce experimental white light LEDs used no phosphors at all and was based on homoepitaxially grown zinc selenide (ZnSe) on a ZnSe substrate which simultaneously emitted blue light from its active region and yellow light from the substrate.[36]
[edit] Organic light-emitting diodes (OLEDs)
If the emitting layer material of the LED is an organic compound, it is known as an Organic Light Emitting Diode (OLED). To function as a semiconductor, the organic emitting material must have conjugated pi bonds. [37] The emitting material can be a small organic molecule in a crystalline phase, or a polymer. Polymer materials can be flexible; such LEDs are known as PLEDs or FLEDs.
Compared with regular LEDs, OLEDs are lighter, and polymer LEDs can have the added benefit of being flexible. Some possible future applications of OLEDs could be:
- Inexpensive, flexible displays
- Light sources
- Wall decorations
- Luminous cloth
OLEDs have been used to produce visual displays for portable electronic devices such as cellphones, digital cameras, and MP3 players. Larger displays have been demonstrated,[38] but their life expectancy is still far too short (<1,000 hours) to be practical[citation needed].
Today, OLEDs operate at substantially lower efficiency than inorganic (crystalline) LEDs.[39]
[edit] Quantum dot LEDs (experimental)
A new technique developed by Michael Bowers, a graduate student at Vanderbilt University in Nashville, involves coating a blue LED with quantum dots that glow white in response to the blue light from the LED. This technique produces a warm, yellowish-white light similar to that produced by incandescent bulbs.[40]
Quantum dots are semiconductor nanocrystals that possess unique optical properties.[41] Their emission color can be tuned from the visible throughout the infrared spectrum. This allows quantum dot LEDs to create almost any color on the CIE diagram. This provides more color options and better color rendering white LEDs. Quantum dot LEDs are available in the same package types as traditional phosphor based LEDs.
[edit] Types
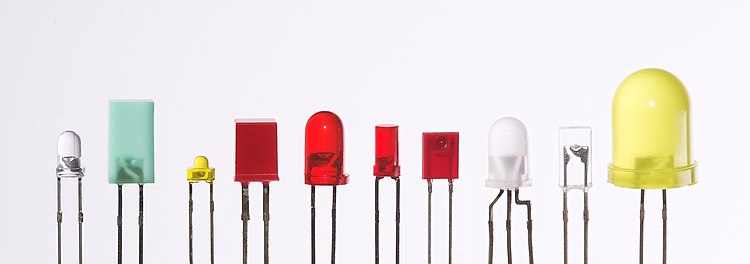
The main types of LEDs are miniature, high power devices and custom designs such as alphanumeric or multi-color.
[edit] Miniature LEDs
These are mostly single-die LEDs used as indicators, and they come in various-sizes from 2 mm to 8 mm, through-hole and surface mount packages. They are usually simple in design, not requiring any separate cooling body.[42] Typical current ratings ranges from around 1 mA to above 20 mA. The small scale set a natural upper boundary on power consumption due to heat caused by the high current density and need for heat sinking.
[edit] High power LEDs
High power LEDs (HPLED) can be driven at hundreds of mA (vs. tens of mA for other LEDs), some with more than one ampere of current, and give out large amounts of light. Since overheating is destructive, the HPLEDs must be highly efficient to minimize excess heat; furthermore, they are often mounted on a heat sink to allow for heat dissipation. If the heat from a HPLED is not removed, the device will burn out in seconds.
A single HPLED can often replace an incandescent bulb in a flashlight, or be set in an array to form a powerful LED lamp.
LEDs have been developed by Seoul Semiconductor that can operate on AC power without the need for a DC converter. For each half cycle part of the LED emits light and part is dark, and this is reversed during the next half cycle. The efficiency of HPLEDs is typically 40 lm/W.[43] Some well-known HPLED's in this category are the Lumileds Rebel Led, Osram Opto Semiconductors Golden Dragon and Cree X-lamp. As of November 2008 some HPLEDs manufactured by Cree Inc. now exceed 95 lm/W [44] (e.g. the XLamp MC-E LED chip emitting Cool White light) and are being sold in lamps intended to replace incandescent, halogen, and even fluorescent style lights as LEDs become more cost competitive.
[edit] Application-specific variations
- Flashing LEDs are used as attention seeking indicators without requiring external electronics. Flashing LEDs resemble standard LEDs but they contain an integrated multivibrator circuit inside which causes the LED to flash with a typical period of one second. In diffused lens LEDs this is visible as a small black dot. Most flashing LEDs emit light of a single color, but more sophisticated devices can flash between multiple colors and even fade through a color sequence using RGB color mixing.

- Bi-color LEDs are actually two different LEDs in one case. It consists of two dies connected to the same two leads but in opposite directions. Current flow in one direction produces one color, and current in the opposite direction produces the other color. Alternating the two colors with sufficient frequency causes the appearance of a blended third color. For example, a red/green LED operated in this fashion will color blend to produce a yellow appearance.
- Tri-color LEDs are two LEDs in one case, but the two LEDs are connected to separate leads so that the two LEDs can be controlled independently and lit simultaneously. A three-lead arrangement is typical with one common lead (anode or cathode).
- RGB LEDs contain red, green and blue emitters, generally using a four-wire connection with one common lead (anode or cathode).
- Alphanumeric LED displays are available in seven-segment and starburst format. Seven-segment displays handle all numbers and a limited set of letters. Starburst displays can display all letters. Seven-segment LED displays were in widespread use in the 1970s and 1980s, but increasing use of liquid crystal displays, with their lower power consumption and greater display flexibility, has reduced the popularity of numeric and alphanumeric LED displays.
[edit] Considerations for use
[edit] Power sources
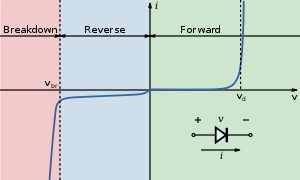
The current/voltage characteristics of an LED is similar to other diodes, in that the current is dependent exponentially on the voltage (see Shockley diode equation). This means that a small change in voltage can lead to a large change in current. If the maximum voltage rating is exceeded by a small amount the current rating may be exceeded by a large amount, potentially damaging or destroying the LED. The typical solution is therefor to use constant current power supplies, or driving the LED at a voltage much below the maximum rating. Since few household power sources (batteries, mains) are constant current sources, most LED fixtures must include a power converter.
[edit] Electrical polarity
| This section needs additional citations for verification. Please help improve this article by adding reliable references (ideally, using inline citations). Unsourced material may be challenged and removed. (March 2009) |
As with all diodes, current flows easily from p-type to n-type material.[45] However, no current flows and no light is produced if a small voltage is applied in the reverse direction. If the reverse voltage becomes large enough to exceed the breakdown voltage, a large current flows and the LED may be damaged.
[edit] Advantages
- Efficiency: LEDs produce more light per watt than incandescent bulbs.[46]
- Color: LEDs can emit light of an intended color without the use of color filters that traditional lighting methods require. This is more efficient and can lower initial costs.
- Size: LEDs can be very small (smaller than 2 mm2[47]) and are easily populated onto printed circuit boards.
- On/Off time: LEDs light up very quickly. A typical red indicator LED will achieve full brightness in microseconds.[48] LEDs used in communications devices can have even faster response times.
- Cycling: LEDs are ideal for use in applications that are subject to frequent on-off cycling, unlike fluorescent lamps that burn out more quickly when cycled frequently, or HID lamps that require a long time before restarting.
- Dimming: LEDs can very easily be dimmed either by Pulse-width modulation or lowering the forward current.
- Cool light: In contrast to most light sources, LEDs radiate very little heat in the form of IR that can cause damage to sensitive objects or fabrics. Wasted energy is dispersed as heat through the base of the LED.
- Slow failure: LEDs mostly fail by dimming over time, rather than the abrupt burn-out of incandescent bulbs.[49]
- Lifetime: LEDs can have a relatively long useful life. One report estimates 35,000 to 50,000 hours of useful life, though time to complete failure may be longer.[50] Fluorescent tubes typically are rated at about 10,000 to 15,000 hours, depending partly on the conditions of use, and incandescent light bulbs at 1,000–2,000 hours.[citation needed]
- Shock resistance: LEDs, being solid state components, are difficult to damage with external shock, unlike fluorescent and incandescent bulbs which are fragile.
- Focus: The solid package of the LED can be designed to focus its light. Incandescent and fluorescent sources often require an external reflector to collect light and direct it in a usable manner.
- Toxicity: LEDs do not contain mercury, unlike fluorescent lamps.
[edit] Disadvantages
- High price: LEDs are currently more expensive, price per lumen, on an initial capital cost basis, than most conventional lighting technologies. The additional expense partially stems from the relatively low lumen output and the drive circuitry and power supplies needed. However, when considering the total cost of ownership (including energy and maintenance costs), LEDs far surpass incandescent or halogen sources and begin to threaten compact fluorescent lamps[citation needed].
- Temperature dependence: LED performance largely depends on the ambient temperature of the operating environment. Over-driving the LED in high ambient temperatures may result in overheating of the LED package, eventually leading to device failure. Adequate heat-sinking is required to maintain long life. This is especially important when considering automotive, medical, and military applications where the device must operate over a large range of temperatures, and is required to have a low failure rate.
- Voltage sensitivity: LEDs must be supplied with the voltage above the threshold and a current below the rating. This can involve series resistors or current-regulated power supplies.[51]
- Light quality: Most cool-white LEDs have spectra that differ significantly from a black body radiator like the sun or an incandescent light. The spike at 460 nm and dip at 500 nm can cause the color of objects to be perceived differently under cool-white LED illumination than sunlight or incandescent sources, due to metamerism,[52] red surfaces being rendered particularly badly by typical phosphor based cool-white LEDs. However, the color rendering properties of common fluorescent lamps are often inferior to what is now available in state-of-art white LEDs.
- Area light source: LEDs do not approximate a “point source” of light, but rather a lambertian distribution. So LEDs are difficult to use in applications requiring a spherical light field. LEDs are not capable of providing divergence below a few degrees. This is contrasted with lasers, which can produce beams with divergences of 0.2 degrees or less.[53]
- Blue Hazard: There is increasing concern that blue LEDs and cool-white LEDs are now capable of exceeding safe limits of the so-called blue-light hazard as defined in eye safety specifications such as ANSI/IESNA RP-27.1-05: Recommended Practice for Photobiological Safety for Lamp and Lamp Systems.[54][55]
- Blue pollution: Because cool-white LEDs (i.e., LEDs with high color temperature) emit much more blue light than conventional outdoor light sources such as high-pressure sodium lamps, the strong wavelength dependence of Rayleigh scattering means that cool-white LEDs can cause more light pollution than other light sources. It is therefore very important that cool-white LEDs are fully shielded when used outdoors. Compared to low-pressure sodium lamps, which emit at 589.3 nm, the 460 nm emission spike of cool-white and blue LEDs is scattered about 2.7 times more by the Earth's atmosphere. Cool-white LEDs should not be used for outdoor lighting near astronomical observatories.
[edit] Applications

The many application of LEDs are very diverse but fall into three major categories: Visual signal application where the light goes more or less directly from the LED to the human eye, to convey a message or meaning. Illumination where LED light is reflected from object to give visual response of these objects. Finally LEDs are also used to generate light for measuring and interacting with processes that do not involve the human visual system.[56]
[edit] Indicators and signs

The low energy consumption, low maintenance and small size of modern LEDs has led to applications as status indicators and displays on a variety of equipment and installations. Large area LED displays are used as stadium displays and as dynamic decorative displays. Thin, lightweight message displays ars used at airports and railway stations, and as destination displays for trains, buses, trams, and ferries.
The single color light is well suited for traffic lights and signals, exit signs, emergency vehicle lighting, ships' lanterns and LED-based Christmas lights. Red or yellow LEDs are used in indicator and alphanumeric displays in environments where night vision must be retained: aircraft cockpits, submarine and ship bridges, astronomy observatories, and in the field, e.g. night time animal watching and military field use.
Because of their long life and fast switching times, LEDs have been used for automotive high-mounted brake lights and truck and bus brake lights and turn signals for some time, but many high-end vehicles are now starting to use LEDs for their entire rear light clusters. The use of LEDs also has styling advantages because LEDs are capable of forming much thinner lights than incandescent lamps with parabolic reflectors. The significant improvement in the time taken to light up (perhaps 0.5s faster than an incandescent bulb) improves safety by giving drivers more time to react. It has been reported that at normal highway speeds this equals one car length increased reaction time for the car behind. White LED headlamps are beginning to make an appearance.
Due to the relative cheapness of low output LEDs they are also used in many temporary applications such as glowsticks and throwies and Lumalive, a photonic textile, artist have also used LEDs for Light art and more specifically LED art.
[edit] Lighting

With the development of high efficiency and high power LEDs it has become possible to incorporate LEDs in lighting and illumination. Replacement light bulbs have been made as well as dedicated fixtures and LED lamps. LEDs are used as street lights and in other architectural lighting where color changing is used. The mechanical robustness and long lifetime is used in automotive lighting on cars, motorcycles and on bicycle lights.
LEDs are also suitable for backlighting for LCD televisions and lightweight laptop displays and light source for DLP projectors. RGB LEDs increase the color gamut by as much as 45%.
The lack of IR/heat radiation makes LEDs ideal for stage lights using banks of RGB LEDs that can easily change color and decrease heating from traditional stage lighting, as well as medical lighting where IR-radiation can be harmful.
Since LEDs are small, durable and require little power they are used in hand held devices such as flashlights. LED strobe lights or camera flashes operate at a safe, low voltage, as opposed to the 250+ volts commonly found in xenon flashlamp-based lighting. This is particularly applicable to cameras on mobile phones, where space is at a premium and bulky voltage-increasing circuitry is undesirable. LEDs are used for infrared illumination in night vision applications including security cameras. A ring of LEDs around a video camera, aimed forward into a retroreflective background, allows chroma keying in video productions.
[edit] Smart lighting
Light can be used to transmit broadband data, which is already implemented in IrDA standards using infrared LEDs. Because LEDs can cycle on and off millions of times per second, they can, in effect, become wireless routers for data transport.[57] Lasers can also be modulated in this manner.
[edit] Non-visual applications
| This section is in a list format that may be better presented using prose. You can help by converting this section to prose, if appropriate. Editing help is available. (March 2009) |

- Grow lights using LEDs to increase photosynthesis in plants[58]
- Remote controls, such as for TVs and VCRs, often use infrared LEDs.
- Movement sensors, for example in optical computer mice. The Nintendo Wii's sensor bar uses infrared LEDs.
- As light sensors
- In optical fiber and Free Space Optics communications.
- In pulse oximeters for measuring oxygen saturation
- Some flatbed scanners use arrays of RGB LEDs rather than the typical cold-cathode fluorescent lamp as the light source. Having independent control of three illuminated colors allows the scanner to calibrate itself for more accurate color balance, and there is no need for warm-up. Furthermore, its sensors only need be monochromatic, since at any one point in time the page being scanned is only lit by a single color of light.
- As UV curing devices for some ink and coating applications as well as LED printers.
- Sterilization of water and other substances using UV light.[32]
- Touch sensing: Since LEDs can also be used as photodiodes, they can be used for both photo emission and detection. This could be used in for example a touch-sensing screen that register reflected light from a finger or stylus.[59]
- Opto-isolators use an LED combined with a photodiode or phototransistor to provide a signal path with electrical isolation between two circuits. This is especially useful in medical equipment where the signals from a low voltage sensor circuit (usually battery powered) in contact with a living organism must be electrically isolated from any possible electrical failure in a recording or monitoring device operating at potentially dangerous voltages. An optoisolator also allows information to be transferred between circuits not sharing a common ground potential.
- LEDs have also been used as a medium quality voltage reference in electronic circuits. The forward voltage drop (e.g., about 1.7 V for a normal red LED) can be used instead of a Zener diode in low-voltage regulators. Although LED forward voltage is much more current-dependent than a good Zener, Zener diodes are not widely available below voltages of about 3 V.
[edit] Light sources for machine vision systems
| This section is in a list format that may be better presented using prose. You can help by converting this section to prose, if appropriate. Editing help is available. (March 2009) |
Machine vision systems often require bright and homogeneous illumination, so features of interest are easier to process.
LEDs are often used to this purpose, and this field of application is likely to remain one of the major application areas until price drops low enough to make signaling and illumination applications more widespread. Barcode scanners are the most common example of machine vision, and many inexpensive ones used red LEDs instead of lasers.
LEDs constitute a nearly ideal light source for machine vision systems for several main reasons:
- Size of illuminated field is usually comparatively small and Vision systems or smart camera are quite expensive, so cost of LEDs is usually a minor concern, compared to signaling applications.
- LED elements tend to be small and can be placed with high density over flat or even shaped substrates (PCBs etc) so that bright and homogeneous sources can be designed which direct light from tightly controlled directions on inspected parts.
- LEDs often have or can be used with small, inexpensive lenses and diffusers, helping to achieve high light densities and very good lighting control and homogeneity.
- LEDs can be easily strobed (in the microsecond range and below) and synchronized; their power also has reached high enough levels that sufficiently high intensity can be obtained, allowing well lit images even with very short light pulses: this is often used in order to obtain crisp and sharp “still” images of quickly-moving parts.
- LEDs come in several different colors and wavelengths, easily allowing to use the best color for each application, where different color may provide better visibility of features of interest. Having a precisely known spectrum allows tightly matched filters to be used to separate informative bandwidth or to reduce disturbing effect of ambient light.
- LEDs usually operate at comparatively low working temperatures, simplifying heat management and dissipation, therefore allowing plastic lenses, filters and diffusers to be used. Waterproof units can also easily be designed, allowing for use in harsh or wet environments (food, beverage, oil industries).
- LED sources can be shaped in several main configurations (spot lights for reflective illumination; ring lights for coaxial illumination; back lights for contour illumination; linear assemblies; flat, large format panels; dome sources for diffused, omnidirectional illumination).
- Very compact designs are possible, allowing for small LED illuminators to be integrated within smart cameras and vision sensors.
[edit] See also
| Look up light-emitting diode in Wiktionary, the free dictionary. |
[edit] References
- Cited
- ^ "LED". http://en.wiktionary.org/wiki/LED. Retrieved on 2008-01-04.
- ^ ... (2008). "Modeling the radiation pattern of LEDs". ... (Optics Express). http://www.opticsexpress.org/viewmedia.cfm?id=149957&seq=0.
- ^ H. J. Round (1907). "A Note on Carborundum". Electrical World 19: 309.
- ^ Zheludev, N. (2007). "The life and times of the LED — a 100-year history" (PDF). Nature Photonics 1 (4): 189–192. doi:. http://www.nanophotonics.org.uk/niz/publications/zheludev-2007-ltl.pdf.
- ^ Margolin J. "The Road to the Transistor". http://www.jmargolin.com/history/trans.htm.
- ^ Braunstein, Rubin (1955.). ""Radiative Transitions in Semiconductors,"". Physical Review 99: 1892-3.. doi:. http://prola.aps.org/abstract/PR/v99/i6/p1892_1.
- ^ "The first LEDs were infrared (invisible)". The Quartz Watch. The Lemelson Center. http://invention.smithsonian.org/centerpieces/quartz/inventors/biard.html. Retrieved on 2007-08-13.
- ^ "Nick Holonyak, Jr. 2004 Lemelson-MIT Prize Winner". Lemenson-MIT Program. http://web.mit.edu/invent/a-winners/a-holonyak.html. Retrieved on 2007-08-13.
- ^ Wolinsky, Howard (February 5, 2005). "U. of I.'s Holonyak out to take some of Edison's luster". Chicago Sun-Times. http://findarticles.com/p/articles/mi_qn4155/is_20050202/ai_n9504926. Retrieved on 2007-07-29.
- ^ Spectrum, IEEE: 52-55. Feb 1995. doi:. ISSN 0018-9235.
- ^ "Brief Biography – Holonyak, Craford, Dupuis" (PDF). Technology Administration. http://www.technology.gov/Medal/2002/bios/Holonyak_Craford_Dupuis.pdf. Retrieved on 2007-05-30.
- ^ T.P. Pearsall, B.I. Miller, R.J. Capik, and K.J. Bachmann, "Efficient, Lattice-matched, Double Heterostructure LEDs at 1.1 mm from GaxIn1-xAsyP1-y by Liquid-phase Epitaxy", Appl. Phys. Lett., 28, 499-501 (1976).
- ^ a b E. Fred Schubert, Light-Emitting Diodes, Cambridge University Press, 2003 Chapter 1
- ^ "LEDs cast Monsanto in Unfamiliar Role". http://eetimes.com/anniversary/designclassics/monsanto.html.
- ^ "LED there be light". http://www.electrooptics.com/features/junjul06/junjul06leds.html. Retrieved on 2009-03-04.
- ^ "The LED Illumination Revolution". http://www.forbes.com/2008/02/27/incandescent-led-cfl-pf-guru_in_mm_0227energy_inl.html. Retrieved on 2009-03-04.
- ^ "LED Thermal Management". http://www.lunaraccents.com/educational-LED-thermal-management.html. Retrieved on march 16 2009.
- ^ "2006 Millennium technology prize awarded to UCSB's Shuji Nakamura". http://www.ia.ucsb.edu/pa/display.aspx?pkey=1475. Retrieved on 2007-05-30.
- ^ "Haitz's law". http://www.nature.com/nphoton/journal/v1/n1/full/nphoton.2006.78.html. Retrieved on 2009-03-04.
- ^ Warm light and high efficiency
- ^ "Seoul Semiconductor squeezes 240 lumens into "brightest" LED". engadget. December 12, 2006. http://www.engadget.com/2006/12/12/seoul-semiconductor-squeezes-240-lumens-into-brightest-led/. Retrieved on 2007-08-13.
- ^ "Nichia Unveils White LED with 150 lm/W Luminous Efficiency". Tech-On!. December 21, 2006. http://techon.nikkeibp.co.jp/english/NEWS_EN/20061221/125713/. Retrieved on 2007-08-13.
- ^ Datasheet is not sufficient to confirm the claim, comparing Philips LXHL-LW6C and OSRAM LUW W5AM-LXLY-6P7R
- ^ CREE (November 19, 2008). Achieves 161 Lumens per Watt from a High-Power LED. Press release. http://www.cree.com/press/press_detail.asp?i=1227101620851. Retrieved on 2009-02-15.
- ^ "Lifetime of White LEDs". http://www1.eere.energy.gov/buildings/ssl/lifetime.html. Retrieved on 2009-02-20.
- ^ "Understanding Power LED Lifetime Analysis". http://www.philipslumileds.com/pdfs/WP12.pdf. Retrieved on 2009-02-15.
- ^ "LEDs move into the ultraviolet". physicsworld.com. May 17, 2006. http://physicsworld.com/cws/article/news/24926. Retrieved on 2007-08-13.
- ^ "Alumni society honors four leaders in engineering and technology". Berkeley Engineering News. 2000-09-04. http://www.coe.berkeley.edu/EPA/EngNews/00F/EN2F/deaa.html. Retrieved on 2007-01-23.
- ^ "GaN-based blue light emitting device development by Akasaki and Amano" (PDF). Takeda Award 2002 Achievement Facts Sheet. The Takeda Foundation. 2002-04-05. http://www.takeda-foundation.jp/en/award/takeda/2002/fact/pdf/fact01.pdf. Retrieved on 2007-11-28.
- ^ "United States Patent No. 5,578,839 (Nakamura et al.)". United States Patent and Trademark Office. filed 1993-11-17. http://patft.uspto.gov/netacgi/nph-Parser?Sect2=PTO1&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=1&f=G&l=50&d=PALL&RefSrch=yes&Query=PN%2F5578839. Retrieved on 2007-01-23.
- ^ Sensor Electronic Technology, Inc.: Nitride Products Manufacturer
- ^ a b Development of a new water sterilization device with a 365 nm UV-LED, Medical and Biological Engineering and Computing, Volume 45, Number 12 / December, 2007
- ^ "Jan Henrik Wold and Arne Valberg". 2001.
- ^ ... (2007). "Color distribution from multicolor LED arrays". ... (Optics Express). http://www.opticsexpress.org/viewmedia.cfm?uri=oe-15-6-3607&seq=0. Retrieved on 2008-09-10.
- ^ "E. Fred Schubert and Jong Kyu Kim". 2005.
- ^ Tim Whitaker (December 6, 2002). "Joint venture to make ZnSe white LEDs". http://optics.org/cws/article/research/16534. Retrieved on 3 January 2009.
- ^ Burroughes, JH and Bradley, DDC and Brown, AR and Marks, RN and Mackay, K. and Friend, RH and Burns, PL and Holmes, AB, (1990). "Light-emitting diodes based on conjugated polymers,". Nature 347 (6293): 539--541.
- ^ Lawler, Richard (2007-01-08). "Sony's 1,000,000:1 contrast ratio 27-inch OLED HDTV". Engadget. http://www.engadget.com/2007/01/08/sonys-1-000-000-1-contrast-ratio-27-inch-oled-hdtv/. Retrieved on 2009-02-15.
- ^ "New study says OLED efficiency is less than previously reported". LEDs Magazine (PennWell Corporation). 2008-08-20. http://www.ledsmagazine.com/news/5/8/18. Retrieved on 2009-02-15.
- ^ "Accidental Invention Points to End of Light Bulbs". LiveScience.com. October 21, 2005. http://www.livescience.com/technology/051021_nano_light.html. Retrieved on 2007-01-24.
- ^ Quantum-dot LED may be screen of choice for future electronics Massachusetts Institute of Technology News Office,December 18, 2002
- ^ LED-design
- ^ "Seoul Semiconductor launches AC LED lighting source Acriche". LEDS Magazine. http://www.ledsmagazine.com/news/3/11/14. Retrieved on 2008-02-17.
- ^ "XLamp® MC-E LED". Cree, Inc. http://www.cree.com/products/xlamp_mce.asp. Retrieved on 2008-11-11.
- ^ E. Fred Schubert (2005). "Chapter 4". Light-Emitting Diodes. Cambridge University Press.
- ^ "Solid-State Lighting: Comparing LEDs to Traditional Light Sources". http://www.netl.doe.gov/ssl/usingLeds/general_illumination_efficiency_comparison.htm.
- ^ "Dialight Micro LED SMD LED “598 SERIES” Datasheet" (PDF). http://www.dialight.com/Assets/Brochures_And_Catalogs/Indication/MDEI5980603.pdf.
- ^ Philips Lumileds technical datasheet DS23 for the Luxeon Star states "less than 100ns".
- ^ "Solid-State Lighting: Lumen Depreciation". http://www.netl.doe.gov/ssl/usingLeds/general_illumination_life_depreciation.htm.
- ^ http://apps1.eere.energy.gov/buildings/publications/pdfs/ssl/lifetime_white_leds_aug16_r1.pdf
- ^ The Led Museum
- ^ James A. Worthey. "How White Light Works". LRO Lighting Research Symposium, Light and Color. http://www.jimworthey.com/jimtalk2006feb.html. Retrieved on 2007-10-06.
- ^ Hecht, E: "Optics", Fourth Edition, page 591. Addison Wesley, 2002.
- ^ "Blue LEDs: A health hazard?". texyt.com. January 15, 2007. http://texyt.com/bright+blue+leds+annoyance+health+risks. Retrieved on 2007-09-03.
- ^ "Light Impacts: Science News Online, May 27, 2006". http://www.sciencenews.org/articles/20060527/bob9.asp. 071214 sciencenews.org
- ^ EPIC European Photonics Industry Consortium.
- ^ Green, Hank (2008-10-09). "Transmitting Data Through LED Light Bulbs". EcoGeek. http://www.ecogeek.org/content/view/2194/74/. Retrieved on 2009-02-15.
- ^ Goins, GD and Yorio, NC and Sanwo, MM and Brown, CS (1997). "Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting". Journal of Experimental Botany (Soc Experiment Biol) 48 (7): 1407. doi:.
- ^ Dietz, Yerazunis, and Leigh (2004). Very Low-Cost Sensing and Communication Using Bidirectional LEDs. http://www.merl.com/publications/TR2003-035/.
- General
- Shuji Nakamura, Gerhard Fasol, Stephen J Pearton The Blue Laser Diode: The Complete Story, Springer Verlag, 2nd Edition (October 2, 2000)
- Mills, Evan (2005). "The Specter of Fuel-Based Lighting". Science 308: 1263–1264. doi:. PMID 15919979.
- Moreno, I., "Spatial distribution of LED radiation," in The International Optical Design Conference, Proc. SPIE vol. 6342, 634216:1-7 (2006).
- Salisbury, David F. (October 20, 2005). "Quantum dots that produce white light could be the light bulb’s successor". Exploration—The Online Research Journal of Vanderbilt University. http://exploration.vanderbilt.edu/news/news_quantumdot_led.htm. (More details regarding the use of quantum dots as a phosphor for white LEDs.)
[edit] External links
| Wikimedia Commons has media related to: Light-emitting diode |
- FlatpanelsHD - review of Philips first LED-based LCD-TV
- Photonics Sources Group, Tyndall National Institute GaN and other photonics research at the Tyndall National Institute, Ireland.
- LEDs for Sustainable Development
- Solid State Lighting, Michael Shur - Rensselaer Polytechnic
- Applications notes about Discrete LEDs including basic driver circuits
- Light emitting diodes.org site
- Dendrimers in the spotlight - an Instant Insight examining the use of dendrimers in organic light-emitting diodes from the Royal Society of Chemistry
- MAKE Presents: The LED - A movie about the origins of the LED and how to make your own from carborundum!
|
|||||||||||||||||||||||||
|
|||||||||||||||||