Biodiesel production
From Wikipedia, the free encyclopedia
Biodiesel production is the act of producing the biofuel, biodiesel, through either transesterification or alcoholysis. The process involves reacting vegetable oils or animal fats catalytically with a short-chain aliphatic alcohols (typically methanol or ethanol).
Contents |
[edit] Transesterification chemistry
A reaction scheme for transesterification is as follows: 
R1, R2, and R3 in this diagram represent long carbon chains that are too lengthy to include in the diagram.
Animal and plant fats and oils are typically made of triglycerides which are esters of free fatty acids with the trihydric alcohol, glycerol. In the transesterification process, the alcohol is deprotonated with a base to make it a stronger nucleophile. Commonly, ethanol or methanol are used. As can be seen, the reaction has no other inputs than the triglyceride and the alcohol.
Normally, this reaction will proceed either exceedingly slowly or not at all. Heat, as well as an acid or base are used to help the reaction proceed more quickly. It is important to note that the acid or base are not consumed by the transesterification reaction, thus they are not reactants but catalysts.
Almost all biodiesel is produced from virgin vegetable oils using the base-catalyzed technique as it is the most economical process for treating virgin vegetable oils, requiring only low temperatures and pressures and producing over 98% conversion yield (provided the starting oil is low in moisture and free fatty acids). However, biodiesel produced from other sources or by other methods may require acid catalysis which is much slower.[1] Since it is the predominant method for commercial-scale production, only the base-catalyzed transesterification process will be described below.
[edit] Steps in the process
The major steps required to synthesize biodiesel are as follows:
[edit] Purification
If waste vegetable oil (WVO) is used, it is filtered to remove dirt, charred food, and other non-oil material often found. Water is removed because its presence causes the triglycerides to hydrolyze to give salts of the fatty acids instead of undergoing transesterification to give biodiesel. At home, this is often accomplished by heating the filtered oil to approximately 120 °C. At this point, dissolved or suspended water will boil off. When the water boils, it spatters (chemists refer to it as "bumping"). To prevent injury, this operation should be done in a sufficiently large container (at most two thirds full) which is closed but not sealed.
In the laboratory, the crude oil may be stirred with a drying agent such as magnesium sulfate to remove the water in the form of water of crystallization. The drying agent can be separated by decanting or by filtration. However, the viscosity of the oil may not allow the drying agent to mix thoroughly.
[edit] Neutralization of free fatty acids
A sample of the cleaned oil is titrated against a standard solution of base in order to determine the concentration of free fatty acids (RCOOH) present in the waste vegetable oil sample. The quantity (in moles) of base required to neutralize the acid is then calculated.
[edit] Transesterification
While adding the base, a slight excess is factored in to provide the catalyst for the transesterification. The calculated quantity of base (usually sodium hydroxide) is added slowly to the alcohol and it is stirred until it dissolves. Sufficient alcohol is added to make up three full equivalents of the triglyceride, and an excess of usually six parts alcohol to one part triglyceride is added to drive the reaction to completion.[2] [3] [4][5]
The solution of sodium hydroxide in the alcohol is then added to a warm solution of the waste oil, and the mixture is heated (typically 50 °C) for several hours (4 to 8 typically) to allow the transesterification to proceed. A condenser may be used to prevent the evaporative losses of the alcohol. Care must be taken not to create a closed system which can explode.
[edit] Final process
The lower layer of the process is composed primarily of glycerine and other waste products. The top layer, a mixture of biodiesel and alcohol, is decanted. The excess alcohol can be distilled off, or it can be extracted with water. If the latter, the biodiesel should be dried by distillation or with a drying agent.
[edit] Reaction
An example of the transesterification reaction equation, shown in skeletal formulas:
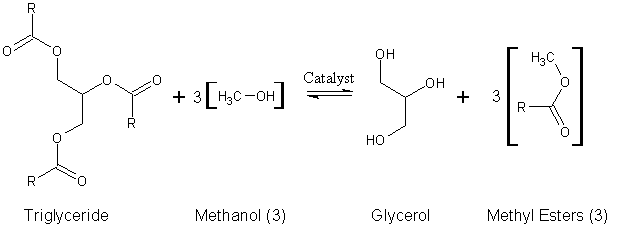
Since natural oils are typically used in this process, the alkyl groups of the triglyceride are not necessarily the same. Therefore, distinguishing these different alkyl groups, we have a more accurate depiction of the reaction:
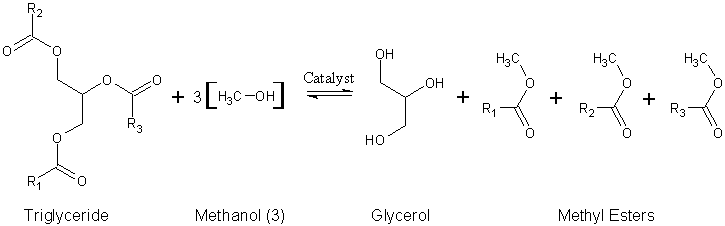
During the esterification process, the triglyceride is reacted with alcohol in the presence of a catalyst, usually a strong alkali (NaOH, KOH, or Alkoxides). The main reason for doing a titration to produce biodiesel, is to find out how much alkaline is needed to completely neutralize any free fatty acids present, thus ensuring a complete transesterification. Empirically 6.25 g / L NaOH produces a very usable fuel. One uses about 6 g NaOH when the WVO is light in colour and about 7 g NaOH when it is dark in colour.
The alcohol reacts with the fatty acids to form the mono-alkyl ester (or biodiesel) and crude glycerol. The reaction between the biolipid (fat or oil) and the alcohol is a reversible reaction so the alcohol must be added in excess to drive the reaction towards the right and ensure complete conversion.
[edit] Base catalysed mechanism
The transesterification reaction is base catalyzed. Any strong base capable of deprotonating the alcohol will do (e.g. NaOH, KOH, Sodium methoxide, etc.). Commonly the base (KOH, NaOH) is dissolved in the alcohol to make a convenient method of dispersing the otherwise solid catalyst into the oil. The ROH needs to be very dry. Any water in the process promotes the saponification reaction, thereby producing salts of fatty acids (soaps) and consuming the base, and thus inhibits the transesterification reaction. Once the alcohol mixture is made, it is added to the triglyceride. The reaction that follows replaces the alkyl group on the triglyceride in a series of steps.
The carbon on the ester of the triglyceride has a slight positive charge, and the carbonyl oxygens have a slight negative charge. This polarization of the C=O bond is what attracts the RO- to the reaction site.
R1
Polarized attraction |
RO- ————————————————> C=O
|
O-CH2-CH-CH2-O-C=O
| |
O-C=O R3
|
R2
This yields a tetrahedral intermediate that has a negative charge on the former carbonyl oxygen:
R1
|
RO-C-O- (pair of electrons)
|
O-CH2-CH-CH2-O-C=O
| |
O-C=O R3
|
R2
These electrons then fall back to the carbon and push off the diacylglycerol forming the ester.
R1
|
RO-C=O
+
-O-CH2-CH-CH2-O-C=O
| |
O-C=O R3
|
R2
Then two more RO groups react via this mechanism at the other two C=O groups. This type of reaction has several limiting factors. RO- has to fit in the space where there is a slight positive charge on the C=O. MeO- works well because it is small in size. As the chain length of the RO- group increases, reaction rates decrease. This effect is called steric hindrance. This effect is a primary reason the short chain alcohols, methanol and ethanol, are typically used.
There are several competing reactions, so care must be taken to ensure the desired reaction pathway occurs. Most methods do this by using an excess of RO-.
The acid-catalyzed method is a slight variant that is also affected by steric hindrance.
[edit] Production methods
[edit] Batch process
- Preparation: care must be taken to monitor the amount of water and free fatty acids in the incoming biolipid (oil or fat). If the free fatty acid level or water level is too high it may cause problems with soap formation (saponification) and the separation of the glycerin by-product downstream.
- Catalyst is dissolved in the alcohol using a standard agitator or mixer.
- The alcohol/catalyst mix is then charged into a closed reaction vessel and the biolipid (vegetable or animal oil or fat) is added. The system from here on is totally closed to the atmosphere to prevent the loss of alcohol.
- The reaction mix is kept just above the boiling point of the alcohol (around 70 °C, 158 °F) to speed up the reaction though some systems recommend the reaction take place anywhere from room temperature to 55 °C (131 °F) for safety reasons. Recommended reaction time varies from 1 to 8 hours; under normal conditions the reaction rate will double with every 10 °C increase in reaction temperature. Excess alcohol is normally used to ensure total conversion of the fat or oil to its esters.
- The glycerin phase is much denser than biodiesel phase and the two can be gravity separated with glycerin simply drawn off the bottom of the settling vessel. In some cases, a centrifuge is used to separate the two materials faster.
- Once the glycerin and biodiesel phases have been separated, the excess alcohol in each phase is removed with a flash evaporation process or by distillation. In other systems, the alcohol is removed and the mixture neutralized before the glycerin and esters have been separated. In either case, the alcohol is recovered using distillation equipment and is re-used. Care must be taken to ensure no water accumulates in the recovered alcohol stream.
- The glycerin by-product contains unused catalyst and soaps that are neutralized with an acid and sent to storage as crude glycerin (water and alcohol are removed later, chiefly using evaporation, to produce 80-88% pure glycerin).
- Once separated from the glycerin, the biodiesel is sometimes purified by washing gently with warm water to remove residual catalyst or soaps, dried, and sent to storage.
[edit] Supercritical process
An alternative, catalyst-free method for transesterification uses supercritical methanol at high temperatures and pressures in a continuous process. In the supercritical state, the oil and methanol are in a single phase, and reaction occurs spontaneously and rapidly. [6] The process can tolerate water in the feedstock, free fatty acids are converted to methyl esters instead of soap, so a wide variety of feedstocks can be used. Also the catalyst removal step is eliminated. [7] High temperatures and pressures are required, but energy costs of production are similar or less than catalytic production routes. [8]
[edit] Ultra- and high-shear in-line and batch reactors
Ultra- and High Shear in-line or batch reactors allow production of biodiesel continuously, semi- continuously, and in batch-mode. This drastically reduces production time and increases production volume. Ultra – Shear, up to three sets of rotor and stator which converts mechanical energy to high tip speed, high shear stress, high shear-frequencies. Droplet size range expected in the low micrometer until sub-micrometer range after one pass.
The reaction takes place in the high-energetic shear zone of the Ultra- and High Shear mixer by reducing the droplet size of the immiscible liquids such as oil or fats and methanol. Therefore, the smaller the droplet size the larger the surface area the faster the catalyst can react.
Ultra- and High Shear mixers are used for the pre-treatment of crude vegetable oil or animal fats, for the transesterification, and water wash process such as:
- Improved De-gumming - Ultra-Shear Mixers are used to rapidly and intimately blend the acid or water into the crude oil, resulting in more efficient removal of phospholipid compounds.
- Improved Refining - The High Shear Mixing action of an single stage high-shear mixer allows less than neutralizing dose of caustic, while still achieving neutralization, dramatically improving Refining Yield, and minimizing soap in the Refined Oil.
- Faster Bleaching - Inline Powder / Liquid high-shear Mixer are capable of mixing the bleaching clay and oil inline, in a single pass.
- Continuous Bio-diesel (Methyl Ester) conversion - Ultra-Shear in-line mixer allows a continuous transesterification process.
- Semi - Continuous Bio-diesel (Methyl Ester) conversion - Multiple-Stage in-line mixer allows a semi-continuous transesterification process within 30 min.
- Batch Bio-diesel (Methyl Ester) conversion - Top-entry high-shear mixer allows a batch transesterification process within 30 min.
- Batch or Continuous Water, Wash process - Reduces amount of water compare to conventional processes. Water amount is reduces to 3% by weight
[edit] Ultrasonic-reactor method
In the ultrasonic reactor method, the ultrasonic waves cause the reaction mixture to produce and collapse bubbles constantly. This cavitation provides simultaneously the mixing and heating required to carry out the transesterification process. Thus using an ultrasonic reactor for biodiesel production drastically reduces the reaction time, reaction temperatures, and energy input. Hence the process of transesterification can run inline rather than using the time consuming batch processing. Industrial scale ultrasonic devices allow for the industrial scale processing of several thousand barrels per day.
[edit] Microwave method
Current research is being directed into using commercial microwave ovens to provide the heat needed in the transesterification process.[4][5] The microwaves provide intense localized heating that may be higher than the recorded temperature of the reaction vessel. A continuous flow process producing 6 liters/minute at a 99% conversion rate has been developed and shown to consume only one-fourth of the energy required in the batch process.[5] Although it is still in the lab-scale, development stage, the microwave method holds great potential to be an efficient and cost-competitive method for commercial-scale biodiesel production.
[edit] See also
[edit] References
- ^ Dubé, Marc A, et al. (2007). “Acid-Catalyzed Transesterification of Canola Oil to Biodiesel under Single- and Two-Phase Reaction Conditions”. Energy & Fuels 21: 2450-2459. American Chemical Society. Retrieved on 2007-11-01.
- ^ Fernando, Sandum D, et al. (2007). “Base-Catalyzed Fast Transesterification of Soybean Oil Using Ultrasonication”. Energy & Fuels 21: 1161-1164. American Chemical Society. Retrieved on 2007-11-01.
- ^ Colucci, José A, et al. (2005). “Biodiesel from an Alkaline Transesterification Reaction of Soybean Oil Using Ultrasonic Mixing”. Journal of American Oil Chemists’ Society 82: 525-530. American Oil Chemists’ Society. Retrieved on 2007-11-01
- ^ a b Leadbetter, Nicholas E, et al. (2006). “Fast, Easy Preparation of Biodiesel Using Microwave Heating”. Energy & Fuels 20: 2281-2283. American Chemical Society. Retrieved on 2007-11-01.
- ^ a b c Leadbetter, Nicholas E, et al. (2007). “Continuous-Flow Preparation of Biodiesel Using Microwave Heating”. Energy & Fuels 21: 1777-1781. American Chemical Society. Retrieved on 2007-11-01.
- ^ Bunkyakiat, Kunchana; Et Al (2006). "Continuous Production of Biodiesel via Transesterification from Vegetable Oils in Supercritical Methanol". Energy and Fuels (American Chemical Society) 20: 812–817. doi:.
- ^ Vera, C.R.; S.A. D'Ippolito, C.L. Pieck, J.M.Parera (2005-08-14). "Production of biodiesel by a two-step supercritical reaction process with adsorption refining" (PDF). 2nd Mercosur Congress on Chemical Engineering, 4th Mercosur Congress on Process Systems Engineering. Retrieved on 2007-12-20.
- ^ Kusdiana, Dadan; Saka, Shiro. "Biodiesel fuel for diesel fuel substitute prepared by a catalyst free supercritical methanol" (PDF). http://www.biodieselgear.com/documentation/Methanol_Super_Critical_Method.pdf. Retrieved on 2007-12-20.
[edit] Further reading
- Carlson, Kimberly Sue, AH4U, 2004
- Gerpen, J.V., Biodiesel processing and production, Fuel Processing Technology, 2005, 86, 1097-1107. doi:10.1016/j.fuproc.2004.11.005
- Ma, F. & Hanna, M.A., Biodiesel production: a review, Bioresource Technology, 1999, 70, 1-15. doi:10.1016/S0960-8524(99)00025-5
[edit] External links
- Fast-Transesterification of Soybean Oil Using Ultrasonication
- Sonochemistry in Transesterification of Biodiesel
- Current State of Ultrasonic Processing for Fast Biodiesel Production
- Biodiesel Production Technology August 2002 - January 2004
- UNL Chemical and Biomolecular Engineering Research and Publications
- Continuous Process for the conversion of vegetable Oils into Methyl Esters of Fatty Acids
- Biodiesel Production Technology
- Analyses of Soybean Biodiesel

