Buspirone
From Wikipedia, the free encyclopedia
 |
|
|
Buspirone
|
|
| Systematic (IUPAC) name | |
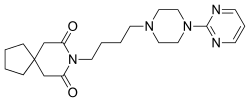
| 8-[4-(4-pyrimidin-2-ylpiperazin-1-yl)butyl]- 8-azaspiro[4.5]decane-7,9-dione |
|
| Identifiers | |
| CAS number | |
| ATC code | N05 |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| Chemical data | |
| Formula | C21H31N5O2 |
| Mol. mass | 385.50314 g/mol |
| Pharmacokinetic data | |
| Bioavailability | low and variable (approx. 5%), due to high first pass metabolism |
| Protein binding | 95% bound to plasma proteins |
| Metabolism | mainly hepatic, active metabolite 1-Pyrimidylpiperazin (1-PP) |
| Half life | 2-3hr |
| Excretion | urine (29-63%) and feces (18-38%) in the form of metabolites |
| Therapeutic considerations | |
| Pregnancy cat. |
B(US) |
| Legal status |
Rx-only, not a controlled substance |
| Routes | oral |
Buspirone (brand-names Ansial, Ansiced, Anxiron, Axoren, Bespar, BuSpar, Buspimen, Buspinol, Buspisal, Narol, Spamilan, Spitomin, Sorbon) is an anxiolytic agent and a serotonin receptor agonist belonging to the azaspirodecanedione class of compounds. Its structure is unrelated to those of the benzodiazepines, but it has an efficacy comparable to diazepam in treating generalized anxiety disorder.[1][2]
It shows no potential for drug addiction or drug tolerance compared to other drugs commonly prescribed for anxiety, especially benzodiazepines. The development of tolerance has not been noticed. Cross-tolerance to benzodiazepines, barbiturates and alcohol does not exist. Furthermore, it is non-sedating.
It is thought to act by altering the brain's serotonin system, particularly by serving as a partial agonist of the 5-HT1A presynaptic receptor. Additionally, it acts as a mixed agonist/antagonist on postsynaptic dopamine receptors. GABA-mediated effects are lacking. Buspirone may also have indirect effects on other neurotransmitters in the brain.
The action of a single dose is much longer than the short half-life of 2-3 hours indicates. The bioavailability of buspirone is very low and variable due to extensive first pass metabolism. The drug is quickly resorbed. Taking the drug together with food may increase the bioavailability. The drug is highly (95%) plasma-bound. The active metabolite 1-PP is also a 5-HT1A partial agonist with anxiolytic properties, but weaker so than the mother-drug.
Buspirone is also useful as an augmenting agent for the treatment of anxiety when added to SSRIs and to potentially lessen their sexual side effects, not unlike bupropion which is an NDRI antidepressant.
The main disadvantage of buspirone is that it takes several weeks before its anxiolytic effects become noticeable. Many patients may also require a higher dosage to adequately respond to treatment, which may also be increased in slow increments of 5 mg every three days and up to 60 mg a day, which may be the dose required for adequate relief. This makes it particularly difficult to treat patients pre-treated with benzodiazepines, for they know the immediate effects of these anxiolytics. Often patients have to be initially co-treated with a benzodiazepine for an immediate anxiolytic effect.
Therefore, benzodiazepines are often the first approach in treating acute panic attacks and social phobias. Although buspirone may be a consideration for patients whose benzodiazepine therapy is becoming extensive beyond weeks, buspirone must not be assumed to counteract the withdrawal effects of benzodiazepines, which in severe chronic high-dose cases, can include seizures, coma and death.
Benzodiazepines should be gradually withdrawn, for example Xanax may safely be withdrawn by .25mg every two weeks in some patients who’ve been taking large chronic doses. As the mechanism of action in the brain between benzodiazepines which antagonise the GABA receptors and buspirone which antagonises the serotonin receptors is uncorrelated, it is essential that buspirone is not considered an anxiolytic agent which may shorten the benzodiazepine withdrawal period or help prevent or lessen the severity of benzodiazepine withdrawal symptoms.
Bristol-Myers Squibb gained FDA approval for Buspirone in 1986. The drug went generic in 2001.
Contents |
[edit] Indications
- Generalized anxiety disorder of mild to moderate intensity (it is not considered effective against other types of anxiety disorders such as obsessive-compulsive disorder, with or without agoraphobia and social phobia)
- Augmentation of SSRI-treatment against depression
- Attention-Deficit Hyperactivity Disorder (ADHD)
- In experimental trials with rats, buspirone has been shown to improve spatial learning and memory after traumatic brain injury (TBI). Such findings may have clinical relevance to TBI patients. [3]
[edit] Contraindications
- Myasthenia gravis
- Acute closed angle glaucoma
- Severely compromised liver- and renal-function
- Concomitant treatment with a MAO-Inhibitor (severe hypertensive crises have been seen)
- Preexisting heart conditions (e.g. myocardial infarction)
[edit] Side-effects
Rarely, side-effects have a dangerous nature or intensity. Some tend to disappear with continued therapy, or are less frequent if the initial dose is low and increased gradually (vertigo, agitation, insomnia).
- Most frequent: vertigo, headaches, nervousness, agitation, light-headedness, nausea;
- Often (>1%) : drowsiness, insomnia, concentration disorders, confusion, depression, agitation, intestinal disorders, paresthesia, coordination disorders, tremors, disturbed vision, tinnitus, fatigue, weakness, angina pectoris, sore throat, tachycardias, palpitations, dry mouth, pain in muscles and joints;
- Seldom: allergic reactions, subdermal bleeding, extrapyramidal symptoms, hallucinations, psychosis, ataxia, epileptic seizures, syncope, tunnel vision, urine retention, hyperosmia, alopecia, pruritus, hot flashes.
There are no dyscognitive side-effects like those seen in benzodiazepines.
Other side-effects have been seen, but are not more frequent than those encountered with placebo. An unusual side effect reported by patients has been an enhanced sense of smell.
[edit] Drug abuse and dependence
Buspirone has no known potential for abuse, psychological or physical dependence.[4]
[edit] Interactions
- Haloperidol : increased plasma-levels of haloperidol
- Rifampicin : decreased plasma-levels of buspirone
- MAO-Inhibitors : severe hypertensive crises are possible.
- Alcohol : The sedative properties of alcohol are increased slightly.
- Grapefruit, Grapefruit juice, Grapefruit extract : drastically increased plasma-levels of Buspirone [5] Substantial quantities of grapefruit juice should be avoided.
[edit] References
- ^ Cohn, JB; Rickels K (1989). "A pooled, double-blind comparison of the effects of buspirone, diazepam and placebo in women with chronic anxiety". Curr Med Res Opin. 11 (5): 304–320.
- ^ Goldberg, HL; Finnerty RJ (September 1979). "The comparative efficacy of buspirone and diazepam in the treatment of anxiety". Am J Psychiatry 136 (9): 1184–1187.
- ^ Kline, Anthony, E., Olsen, Adam, S., Zafonte, Ross D., Sozda, Christopher N., Aslam, Haris, A., and Cheng, Jeffrey P. (September 2007). "Brain injury delayed and chronic buspirone treatment after experimental traumatic brain injury enhances spatial acquisition". Archives of Physical Medicine and Rehabilitation. 88 (9): E6.
- ^ Lydiurd, R. Bruce (2000). "An Overview of Generalized Anxiety Disorder: Disease State-Appropriate Therapy". Clinical Therapeutics 22 (Supplement A): A3–A24. doi:.
- ^ Lilja, JJ; Kivisto KT, Backman JT, Lamberg TS, Neuvonen PJ (December 1998). "Grapefruit juice substantially increases plasma concentrations of buspirone". Clinical Pharmacology & Therapeutics 64 (6): 655–660.
[edit] External Links
Official BuSpar website (Site is under construction as of January 6th, 2009)
|
|||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||

