Vitamin D
From Wikipedia, the free encyclopedia

Vitamin D is a group of fat-soluble prohormones, the two major forms of which are vitamin D2 (or ergocalciferol) and vitamin D3 (or cholecalciferol).[1] The term vitamin D also refers to metabolites and other analogues of these substances. Vitamin D3 is produced in skin exposed to sunlight, specifically ultraviolet B radiation.
Vitamin D plays an important role in the maintenance of organ systems.[2]
- Vitamin D regulates the calcium and phosphorus levels in the blood by promoting their absorption from food in the intestines, and by promoting re-absorption of calcium in the kidneys, which enables normal mineralization of bone and prevents hypocalcemic tetany. It is also needed for bone growth and bone remodeling by osteoblasts and osteoclasts.[3][4].
- In the absence of vitamin K or with drugs (particularly blood thinners) that interfere with Vitamin K metabolism, Vitamin D can promote soft tissue calcification.[5]
- It inhibits parathyroid hormone secretion from the parathyroid gland.[6][7]
- Vitamin D affects the immune system by promoting phagocytosis, anti-tumor activity, and immunomodulatory functions. [8]
Vitamin D deficiency can result from inadequate intake coupled with inadequate sunlight exposure, disorders that limit its absorption, conditions that impair conversion of vitamin D into active metabolites, such as liver or kidney disorders, or, rarely, by a number of hereditary disorders.[2] Deficiency results in impaired bone mineralization, and leads to bone softening diseases, rickets in children and osteomalacia in adults, and possibly contributes to osteoporosis. However, sunlight exposure, to avoid deficiency, carries other risks, including skin cancer; this risk is avoided with dietary absorption, either through diet or as a dietary supplement.
Contents |
[edit] Forms
| Name | Chemical composition | Structure |
| Vitamin D1 | molecular compound of ergocalciferol with lumisterol, 1:1 | |
| Vitamin D2 | ergocalciferol (made from ergosterol) | 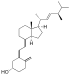 |
| Vitamin D3 | cholecalciferol (made from 7-dehydrocholesterol in the skin). |  |
| Vitamin D4 | 22-dihydroergocalciferol |  |
| Vitamin D5 | sitocalciferol (made from 7-dehydrositosterol |  |
Several forms (vitamers) of vitamin D have been discovered (see table). The two major forms are vitamin D2 or ergocalciferol, and vitamin D3 or cholecalciferol. These are known collectively as calciferol.[9]
Chemically, the various forms of vitamin D are secosteroids; i.e., steroids in which one of the bonds in the steroid rings is broken.[10] The structural difference between vitamin D2 and vitamin D3 is in their side chains. The side chain of D2 contains a double bond between carbons 22 and 23, and a methyl group on carbon 24.
Vitamin D2 (made from ergosterol) is produced by invertebrates, fungus and plants in reponse to UV irradiation; it is not produced by vertebrates. Little is known about the biologic function of vitamin D2 in nonvertebrate species. Because ergosterol can more efficiently absorb the ultraviolet radiation that can damage DNA, RNA, and protein it has been suggested that ergosterol serves as a sunscreening system that protects organisms from damaging high energy ultraviolet radiation.[11]
Vitamin D3 is made in the skin when 7-dehydrocholesterol reacts with UVB ultraviolet light at wavelengths between 270–300 nm, with peak synthesis occurring between 295-297 nm.[12][13] These wavelengths are present in sunlight when the UV index is greater than 3. At this solar elevation, which occurs daily within the tropics, daily during the spring and summer seasons in temperate regions, and almost never within the arctic circles, adequate amounts of vitamin D3 can be made in the skin after only ten to fifteen minutes of sun exposure at least two times per week to the face, arms, hands, or back without sunscreen. With longer exposure to UVB rays, an equilibrium is achieved in the skin, and the vitamin simply degrades as fast as it is generated.[1]
In humans, D3 is as effective as D2 at increasing the levels of vitamin D hormone in circulation,[14] although others state that D3 is more effective than D2.[15] However, in some species, such as rats, vitamin D2 is more effective than D3.[16] Both vitamin D2 and D3 are used for human nutritional supplementation, and pharmaceutical forms include calcitriol (1alpha, 25-dihydroxycholecalciferol), doxercalciferol and calcipotriene.[17]
[edit] Biochemistry
Vitamin D is a prohormone, meaning that it has no hormone activity itself, but is converted to the active hormone 1,25-D through a tightly regulated synthesis mechanism. Production of vitamin D in nature always appears to require the presence of some UV light; even vitamin D in foodstuffs is ultimately derived from organisms, from mushrooms to animals, which are not able to synthesize it except through the action of sunlight at some point in the synthetic chain. For example, fish contain vitamin D only because they ultimately exist on calories from ocean algae which synthesize vitamin D in shallow waters from the action of solar UV.
[edit] Production in the skin
The skin consists of two primary layers: the inner layer called the dermis, composed largely of connective tissue, and the outer, thinner epidermis. The epidermis consists of five strata; from outer to inner they are: the stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale.
Vitamin D3 is produced photochemically in the skin from 7-dehydrocholesterol. 7-Dehydrocholesterol is produced in relatively large quantities in the skin of most vertebrate animals, including humans. The few exceptions are some bat species, mole rats, cats, and dogs,[11] accordingly these animals produce little vitamin D.[18] In most animals the highest concentrations of 7-dehydrocholesterol are found in the epidermal layer of skin, specifically in the stratum basale and stratum spinosum.[12] The production of pre-vitamin D3 is therefore greatest in these two layers, whereas production in the other layers is less. At room temperature the transformation of previtamin-D3 to vitamin D3 takes about 12 days to complete.[11]
Synthesis in the skin involves UVB radiation which effectively penetrates only the epidermal layers of skin. While 7-Dehydrocholesterol absorbs UV light at wavelengths between 270–300 nm, optimal synthesis occurs in a narrow band of UVB spectra between 295-300 nm. Peak isomerization is found at 297 nm. This narrow segment is sometimes referred to as D-UV.[13] The two most important factors that govern the generation of pre-vitamin D3 are the quantity (intensity) and quality (appropriate wavelength) of the UVB irradiation reaching the 7-dehydrocholesterol deep in the stratum basale and stratum spinosum.[12]
A critical determinant of vitamin D3 production in the skin is the presence and concentration of melanin. Melanin functions as a light filter in the skin, and therefore the concentration of melanin in the skin is related to the ability of UVB light to penetrate the epidermal strata and reach the 7-dehydrocholesterol-containing stratum basale and stratum spinosum. Under normal circumstances, ample quantities of 7-dehydrocholesterol (about 25-50 µg/cm² of skin) are available in the stratum spinosum and stratum basale of the skin to meet the body's vitamin D requirements,[12] and melanin content does not alter the amount of vitamin D that can be produced.[19] Thus, individuals with higher skin melanin content will simply require more time in sunlight to produce the same amount of vitamin D as individuals with lower melanin content. As noted below, the amount of time an individual requires to produce a given amount of Vitamin D may also depend upon the person's distance from the equator and on the season of the year.
In some animals the presence of fur or feathers blocks the UV rays from reaching the skin. In birds and fur-bearing mammals vitamin D is generated from the oily secretions of the skin deposited onto the fur and obtained orally during grooming.[20]
[edit] Synthesis mechanism (form 3)
| Vitamin D3 is synthesized from 7-dehydrocholesterol, a derivative of cholesterol, which is then photolyzed by ultraviolet light in 6-electron conrotatory electrocyclic reaction. The product is pre-vitamin D3. | |
| Pre-vitamin D3 then spontaneously isomerizes to Vitamin D3 in a antarafacial hydride [1,7] Sigmatropic shift. | |
| Whether it is made in the skin or ingested, vitamin D3 (cholecalciferol) is then hydroxylated in the liver to 25-hydroxycholecalciferol (25(OH)D3 or calcidiol) by the enzyme 25-hydroxylase produced by hepatocytes, and stored until it is needed.
25-hydroxycholecalciferol is further hydroxylated in the kidneys by the enzyme 1α-hydroxylase, into two dihydroxylated metabolites, the main biologically active hormone 1,25-dihydroxycholecalciferol (1,25(OH)2D3 or calcitriol) and 24R,25(OH)2D3. This conversion occurs in a tightly regulated fashion, with renal 1α-hydroxylase being stimulated by either parathyroid hormone or hypophosphatemia. Calcitriol is represented below right (hydroxylated Carbon 1 is on the lower ring at right, hydroxylated Carbon 25 is at the upper right end). |
[edit] Mechanism of action
After vitamin D is produced in the skin or consumed in food, it is converted in the liver and kidney to form 1,25 dihydroxyvitamin D, (1,25(OH)2D) the physiologically active form of vitamin D (when "D" is used without a subscript it refers to either D2 or D3). This metabolically active form of vitamin D is known as calcitriol. Following this conversion, calcitriol is released into the circulation, and by binding to a carrier protein in the plasma, vitamin D binding protein (VDBP), it is transported to various target organs.[10]
The hormonally active form of vitamin D mediates its biological effects by binding to the vitamin D receptor (VDR), which is principally located in the nuclei of target cells.[10] The binding of calcitriol to the VDR allows the VDR to act as a transcription factor that modulates the gene expression of transport proteins (such as TRPV6 and calbindin), which are involved in calcium absorption in the intestine.
The Vitamin D receptor belongs to the nuclear receptor superfamily of steroid/thyroid hormone receptors, and VDR are expressed by cells in most organs, including the brain, heart, skin, gonads, prostate, and breast. VDR activation in the intestine, bone, kidney, and parathyroid gland cells leads to the maintenance of calcium and phosphorus levels in the blood (with the assistance of parathyroid hormone and calcitonin) and to the maintenance of bone content.[21]
The VDR is known to be involved in cell proliferation and differentiation. Vitamin D also affects the immune system, and VDR are expressed in several white blood cells including monocytes and activated T and B cells.[17]
[edit] Nutrition

A blood calcidiol (25-hydroxy-vitamin D) level is the accepted way to determine vitamin D nutritional status. The optimal level of serum 25-hydroxyvitamin D is 35–55 ng/mL; with some debate among medical scientists for the slightly higher value.[22]
Vitamin D is naturally produced by the human body when exposed to direct sunlight. Season, geographic latitude, time of day, cloud cover, smog, and sunscreen affect UV ray exposure and vitamin D synthesis in the skin, and it is important for individuals with limited sun exposure to include good sources of vitamin D in their diet. Extra vitamin D is also recommended for older adults and people with dark skin. Individuals with a high-risk of deficiency should consume 25 μg (1000 IU) of vitamin D daily to maintain adequate blood concentrations of 25-hydroxyvitamin D.[1]
As civilization and the Industrial Revolution enabled humans to work indoors and wear more clothes when in the sun, these cultural changes reduced natural production of vitamin D and caused deficiency diseases. In many countries, foods such as milk, yogurt, margarine, oil spreads, breakfast cereal, pastries, and bread are fortified with vitamin D2 and/or vitamin D3, to minimize the risk of vitamin D deficiency.[23] In the United States and Canada, for example, fortified milk typically provides 100 IU per glass, or one quarter of the estimated adequate intake for adults over the age of 50.[1] Supplementation of 100 IU (2.5 microgram) vitamin D3 raises blood calcidiol levels by 2.5 nmol/litre (1 ng/ml).[22]
Natural sources of vitamin D include:[1]
- Fish liver oils, such as cod liver oil, 1 Tbs. (15 mL) provides 1,360 IU (one IU equals 25 ng)
- Fatty fish species, such as:
- Herring, 85 g (3 ounces (oz)) provides 1383 IU
- Catfish, 85 g (3 oz) provides 425 IU
- Salmon, cooked, 100 g (3.5 oz]) provides 360 IU
- Mackerel, cooked, 100 g (3.5 oz]), 345 IU
- Sardines, canned in oil, drained, 50 g (1.75 oz), 250 IU
- Tuna, canned in oil, 85 g (3 oz), 200 IU
- Eel, cooked, 100 g (3.5 oz), 200 IU
- One whole egg, provides 20 IU
- Beef liver, cooked, 100 g (3.5 oz), provides 15 IU
- Sun-dried shiitake mushrooms
[edit] Deficiency
Deficiency of vitamin D can result from a number of factors including: inadequate intake coupled with inadequate sunlight (UVB) exposure, disorders that limit its absorption from the gastrointestinal tract, conditions that impair conversion of vitamin D into active metabolites, such as liver or kidney disorders and body characteristics such as skin color and body fat. Rarely deficiency can result from a number of hereditary disorders.[2] Deficiency results in impaired bone mineralization, and leads to bone softening diseases[24] including:
- Rickets, a childhood disease characterized by impeded growth, and deformity, of the long bones. The role of diet in the development of rickets was determined by Edward Mellanby between 1918–1920.[25] In 1921 Elmer McCollum identified a substance found in certain fats that could prevent rickets. Prior to the fortification of milk products with vitamin D, rickets was a major public health problem. In the United States the fortification of milk with 10 micrograms (400 IU) of vitamin D per quart in the 1930s led to a dramatic decline in the number of rickets cases.[21]
- Osteomalacia, a bone-thinning disorder that occurs exclusively in adults and is characterized by proximal muscle weakness and bone fragility. The effects of osteomalacia are thought to contribute to chronic musculoskeletal pain.[26] A number of reports thus indicate that vitamin D deficiency may be related to various types of pain,[27] but of the five small double-blind randomized controlled trials, only one found a reduction in pain after supplementation, and there is no persuasive evidence of lower vitamin D status in chronic pain sufferers compared to controls.[28]
- Osteoporosis, a condition characterized by reduced bone mineral density and increased bone fragility.
Vitamin D malnutrition may also be linked to an increased susceptibility to several chronic diseases such as high blood pressure, tuberculosis, cancer, periodontal disease, multiple sclerosis, chronic pain, seasonal affective disorder [29][30], peripheral artery disease[31], cognitive impairment which includes memory loss and foggy brain,[32] and several autoimmune diseases including type 1 diabetes (see role in immunomodulation).[21][8] There is an association between low vitamin D levels and Parkinson's disease, but whether Parkinson's causes low vitamin D levels, or whether low vitamin D levels play a role in the pathogenesis of Parkinson's disease has not been established.[33]
[edit] Overdose
Vitamin D stored in the human body as calcidiol (25-hydroxy-vitamin D) has a large volume of distribution and a half-life of about 20 to 29 days.[17] Ordinarily, the synthesis of bioactive vitamin D hormone is tightly regulated, and prevalent thinking is that vitamin D toxicity usually only occurs only if excessive doses (prescription forms or rodenticide analogs) are taken.[34] Serum levels of calcidiol (25-hydroxy-vitamin D) are typically used to diagnose vitamin D overdose. In healthy individuals, calcidiol levels are normally between 32 to 70 ng/mL (80 to 175 nmol/L), but these levels may be as much as 15-fold greater in cases of vitamin D toxicity. Serum levels of bioactive vitamin D hormone (1,25(OH2)D) are usually normal in cases of vitamin D overdose.[2]
The exact long-term safe dose of vitamin D is not known. In 1997 the U.S. Dietary Reference Intake Tolerable Upper Intake Level (UL) of vitamin D for children and adults was set at 50 micrograms/day (2,000 IU), but this is viewed as outdated and overly restrictive. A 2007 risk assessment suggested that 250 micrograms/day (10,000 IU) in healthy adults should be adopted as the tolerable upper limit.[35] In adults, sustained intake of 2500 micrograms/day (100,000 IU) can produce toxicity within a few months.[2] For infants (birth to 12 months) the tolerable UL is set at 25 micrograms/day (1000 IU), and vitamin D concentrations of 1000 micrograms/day (40,000 IU) in infants has been shown to produce toxicity within 1 to 4 months. Other sources indicate that the threshold for vitamin D toxicity in humans is 500 to 600 micrograms per kilogram body weight per day."[36] In rats an oral LD50 of 619 mg/kg is noted.[37] All known cases of vitamin D toxicity with hypercalcemia have involved intake of or over 1,000 micrograms/day (40,000 IU)[38].
Although normal food and pill vitamin D concentration levels are far too low to be toxic in adults, because of the high vitamin A content in codliver oil, it is possible to reach toxic levels of vitamin A (but not vitamin D) via this route, [39] if taken in multiples of the normal dose in an attempt to increase the intake of vitamin D. Thus, most officially-recorded historical cases of vitamin D overdose have occurred due to manufacturing and industrial accidents.[38] In the United States, overdose exposure of vitamin D was reported by 284 individuals in 2004, leading to 1 death.[40]
Some symptoms of vitamin D toxicity are a result of hypercalcemia (an elevated level of calcium in the blood) caused by increased intestinal calcium absorption. Vitamin D toxicity is known to be a cause of high blood pressure.[41] Gastrointestinal symptoms of vitamin D toxicity can include anorexia, nausea, and vomiting. These symptoms are often followed by polyuria (excessive production of urine), polydipsia (increased thirst), weakness, nervousness, pruritus (itch), and eventually renal failure. Other signals of kidney disease including elevated protein levels in the urine, urinary casts, and a build up of wastes in the blood stream can also develop.[2] In one study, hypercalciuria and bone loss occurred in four patients with documented vitamin D toxicity.[42] Another study showed elevated risk of ischaemic heart disease when 25D was above 89 ng/mL.[43] Vitamin D toxicity is treated by discontinuing vitamin D supplementation, and restricting calcium intake. If the toxicity is severe blood calcium levels can be further reduced with corticosteroids or bisphosphonates. In some cases kidney damage may be irreversible.[2]
Exposure to sunlight for extended periods of time does not normally cause vitamin D toxicity.[38] This is because within about 20 minutes of ultraviolet exposure in light skinned individuals (3–6 times longer for pigmented skin) the concentration of vitamin D precursors produced in the skin reach an equilibrium, and any further vitamin D that is produced is degraded.[44] Maximum endogenous production with full body exposure to sunlight is 250 µg (10,000 IU) per day.[38]
[edit] Role in immunomodulation
The hormonally active form of vitamin D mediates immunological effects by binding to nuclear vitamin D receptors (VDR) which are present in most immune cell types including both innate and adaptive immune cells. The VDR is expressed constitutively in monocytes and in activated macrophages, dendritic cells, NK cells, T and B cells. In line with this observation, activation of the VDR has potent anti-proliferative, pro-differentiative, and immunomodulatory functions including both immune-enhancing and immunosuppressive effects.[45]
VDR ligands have been shown to increase the activity of natural killer cells, and enhance the phagocytic activity of macrophages.[17] Active vitamin D hormone also increases the production of cathelicidin, an antimicrobial peptide that is produced in macrophages triggered by bacteria, viruses, and fungi.[46] Vitamin D deficiency tends to increase the risk of infections, such as influenza[47] and tuberculosis[48][49][50]. In a 1997 study, Ethiopian children with rickets were 13 times more likely to get pneumonia than children without rickets.[51]
Effects of VDR-ligands, such as vitamin D hormone, on T-cells include suppression of T cell activation and induction of regulatory T cells, as well as effects on cytokine secretion patterns.[52] VDR-ligands have also been shown to affect maturation, differentiation, and migration of dendritic cells, and inhibits DC-dependent T cell activation, resulting in an overall state of immunosuppression.[53]
These immunoregulatory properties indicate that ligands with the potential to activate the VDR, including supplementation with calcitriol (as well as a number of synthetic modulators), may have therapeutic clinical applications in the treatment of; inflammatory diseases (rheumatoid arthritis, psoriatic arthritis), dermatological conditions (psoriasis, actinic keratosis), osteoporosis, cancers (prostate, colon, breast, myelodysplasia, leukemia, head and neck squamous cell carcinoma, and basal cell carcinoma), and autoimmune diseases (systemic lupus erythematosus, type I diabetes; central nervous systems diseases (multiple sclerosis); and in preventing organ transplant rejection.[45]
A 2006 study published in the Journal of the American Medical Association, reported evidence of a link between Vitamin D deficiency and the onset of multiple sclerosis; the authors posit that this is due to the immune-response suppression properties of Vitamin D.[54] Further research indicates that vitamin D is required to activate a histocompatibility gene (HLA-DRB1*1501) necessary for differentiating between self and foreign proteins in a subgroup of individuals genetically predisposed to MS.[55]
[edit] Role in cancer prevention and recovery
The vitamin D hormone, calcitriol, has been found to induce death of cancer cells in vitro and in vivo. The anti-cancer activity of vitamin D is thought to result from its role as a nuclear transcription factor that regulates cell growth, differentiation, apoptosis and a wide range of cellular mechanisms central to the development of cancer.[56] These effects may be mediated through vitamin D receptors expressed in cancer cells.[17]
A search of primary and review medical literature published between 1970 and 2007 found an increasing body of research supporting the hypothesis that the active form of vitamin D has significant, protective effects against the development of cancer. Epidemiological studies show an inverse association between sun exposure, serum levels of 25(OH)D, and intakes of vitamin D and risk of developing and/or surviving cancer. In 2005, scientists released a metastudy which demonstrated a beneficial correlation between vitamin D intake and prevention of cancer. Drawing from a meta-analysis of 63 published reports, the authors showed that intake of an additional 1,000 international units (IU) (or 25 micrograms) of vitamin D daily reduced an individual's colon cancer risk by 50%, and breast and ovarian cancer risks by 30%.[57][58][59] A scientific review undertaken by the National Cancer Institute found that vitamin D was beneficial in preventing colorectal cancer, which showed an inverse relationship with blood levels of 80 nmol/L or higher associated with a 72% risk reduction. However, the same study found no link between baseline vitamin D status and overall cancer mortality.[60]
A 2006 study using data on over 4 million cancer patients from 13 different countries showed a marked difference in cancer risk between countries classified as sunny and countries classified as less–sunny for a number of different cancers.[61] Research has also suggested that cancer patients who have surgery or treatment in the summer — and therefore make more endogenous vitamin D — have a better chance of surviving their cancer than those who undergo treatment in the winter when they are exposed to less sunlight.[62] Another 2006 study found that taking the U.S. RDA of vitamin D (400 IU per day) cut the risk of pancreatic cancer by 43% in a sample of more than 120,000 people from two long-term health surveys.[63][64] A randomized intervention study involving 1,200 women, published in June 2007, reports that vitamin D supplementation (1,100 international units (IU)/day) resulted in a 60% reduction in cancer incidence, during a four-year clinical trial, rising to a 77% reduction for cancers diagnosed after the first year (and therefore excluding those cancers more likely to have originated prior to the vitamin D intervention).[65][66] Research has also indicated beneficial effects of high levels of calcitriol on patients with advanced prostate cancer.[67]
Low levels of vitamin D in serum have also been correlated with breast cancer disease progression and bone metastases,[68] and studies suggest that increased intake of vitamin D reduces the risk of breast cancer in premenopausal women.[69] Polymorphisms of the vitamin D receptor (VDR) gene have been associated with an increased risk of breast cancer.[68] Impairment of the VDR-mediated gene expression is though to alter mammary gland development or function and may predispose cells to malignant transformation. Women with homozygous FOK1 mutations in the VDR gene had an increased risk of breast cancer compared with the women who did not. FOK1 mutation has also been associated with decreasing bone mineral density which in turn may be associated with an increase in the risk of breast cancer.[70]
[edit] Role in cardiovascular disease prevention
Research indicates that vitamin D may play a role in preventing or reversing coronary disease.[71][72] Vitamin D deficiency is associated with an increase in high blood pressure and cardiovascular risk. When researchers monitored the vitamin D levels, blood pressure and other cardiovascular risk factors of 1739 people, of an average age of 59 years for 5 years, they found that those people with low levels of vitamin D had a 62% higher risk of a cardiovascular event than those with normal vitamin D levels.[73] Low levels of vitamin D have also been implicated in hypertension, elevated VLDL triglycerides, and impaired insulin metabolism.[74]
A report from the National Health and Nutrition Examination Survey (NHANES) involving nearly 5,000 participants found that low levels of vitamin D were associated with an increased risk of peripheral artery disease (PAD). The incidence of PAD was 80% higher in participants with the lowest vitamin D levels (<17.8 ng/mL).[31] Cholesterol levels were found to be reduced in gardeners in the UK during the summer months.[75] Heart attacks peak in winter and decline in summer in temperate[76] but not tropical latitudes.[77]
The issue of vitamin D in heart health has not yet been settled, and exercise may account for some of the benefit attributed to vitamin D, since vitamin D levels are generally higher in physically active persons.[78] Moreover, there may be an upper limit after which cardiac benefits decline. One study found an elevated risk of ischaemic heart disease in Southern India in individuals whose vitamin D levels were above 89 ng/mL.[43] These sun-living groups results do not generalize to sun-deprived urban dwellers. Among a group with heavy sun exposure, taking supplemental vitamin D is unlikely to result in blood levels over the ideal range, while urban dwellers not taking supplemental vitamin D may fall under the levels recognized as ideal.
[edit] Role in all-cause mortality
Using information from the National Health and Nutrition Examination Survey a group of researchers concluded that having low levels of vitamin D (<17.8 ng/mL) was independently associated with an increase in all-cause mortality in the general population.[79] The study evaluated whether low serum vitamin D levels were associated all-cause mortality, cancer, and cardiovascular disease (CVD) mortality among 13,331 diverse American adults who were 20 years or older. Vitamin D levels of these participants were collected over a 6-year period (from 1988 through 1994), and individuals were passively followed for mortality through the year 2000.
Among many factors that may be responsible for vitamin D's apparent beneficial effect on all-cause mortality is its effect on telomeres and its potential effect on slowing aging. Shortening of leukocyte telomeres is a marker of aging. Leukocyte telomere length (LTL) predicts the development of aging-related disease, and length of these telomeres decreases with each cell division and with increased inflammation (more common in the elderly) Research indicates that vitamin D is a potent inhibitor of the proinflammatory response and slows the turnover of leukocytes. Higher vitamin D levels were also associated with longer leukocyte telomere length, indicating that vitamin D sufficiency may be play a role in preventing age-related diseases.[80]
[edit] References
- ^ a b c d e "Dietary Supplement Fact Sheet: Vitamin D". National Institutes of Health. Archived from the original on 2007-09-10. http://www.webcitation.org/5Rl5u0LB5. Retrieved on 2007-09-10.
- ^ a b c d e f g Vitamin D at Merck Manual of Diagnosis and Therapy Professional Edition
- ^ van den Berg H (January 1997). "Bioavailability of vitamin D". Eur J Clin Nutr 51 Suppl 1: S76–9. PMID 9023488.
- ^ Cranney A, Horsley T, O'Donnell S, et al (August 2007). "Effectiveness and safety of vitamin D in relation to bone health". Evid Rep Technol Assess (Full Rep) (158): 1–235. PMID 18088161.
- ^ Price PA, Faus SA, Williamson MK (February 2000). "Warfarin-induced artery calcification is accelerated by growth and vitamin D". Arterioscler. Thromb. Vasc. Biol. 20 (2): 317–27. PMID 10669626. http://atvb.ahajournals.org/cgi/pmidlookup?view=long&pmid=10669626.
- ^ Silver J, Russell J, Sherwood LM (June 1985). "Regulation by vitamin D metabolites of messenger ribonucleic acid for preproparathyroid hormone in isolated bovine parathyroid cells". Proc. Natl. Acad. Sci. U.S.A. 82 (12): 4270–3. doi:. PMID 3858880. PMC: 397979. http://www.pnas.org/cgi/pmidlookup?view=long&pmid=3858880.
- ^ Silver J, Naveh-Many T, Mayer H, Schmelzer HJ, Popovtzer MM (November 1986). "Regulation by vitamin D metabolites of parathyroid hormone gene transcription in vivo in the rat". J. Clin. Invest. 78 (5): 1296–301. doi:. PMID 3771798.
- ^ a b Travera-Mendoza, Luz E. and White, John H. "Cell Defenses and the Sunshine Vitamin." Scientific American, November 2007, p. 42.
- ^ Dorland's Illustrated Medical Dictionary, under Vitamin (Table of Vitamins)
- ^ a b c About Vitamin D Including Sections: History, Nutrition, Chemistry, Biochemistry, and Diseases. University of California Riverside
- ^ a b c Holick MF (March 2004). "Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis". Am. J. Clin. Nutr. 79 (3): 362–71. PMID 14985208. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=14985208.
- ^ a b c d Norman AW (June 1998). "Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxyvitamin D: integral components of the vitamin D endocrine system". Am. J. Clin. Nutr. 67 (6): 1108–10. PMID 9625080. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=9625080.
- ^ a b MacLaughlin JA, Anderson RR, Holick MF (May 1982). "Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin". Science (journal) 216 (4549): 1001–3. PMID 6281884. http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=6281884.
- ^ Holick MF, Biancuzzo RM, Chen TC, et al (March 2008). "Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D". J. Clin. Endocrinol. Metab. 93 (3): 677–81. doi:. PMID 18089691.
- ^ Institute of Medicine. (2006) Dietary Reference Intakes Research Synthesis: Workshop Summary, p. 27. National Academies Press.
- ^ Coates, M. E. (1968). "Requirements of different species for vitamins Full Text-pdf". Proceedings of the Nutrition Society 27 (2): 143–148. doi:. PMID 5755261. http://docstore.ingenta.com/cgi-bin/ds_deliver/1/u/d/ISIS/33840020.1/cabi/pns/1968/00000027/00000002/art00019/89A25EAB89BAB011116525855554705D47CC65A51B.pdf?link=http://www.ingentaconnect.com/error/delivery&format=pdf.
- ^ a b c d e Vitamin D; The Physicians Desk Reference. 2006 Thompson Healthcare.
- ^ How KL, Hazewinkel HA, Mol JA (October 1994). "Dietary vitamin D dependence of cat and dog due to inadequate cutaneous synthesis of vitamin D". Gen. Comp. Endocrinol. 96 (1): 12–8. doi:. PMID 7843559.
- ^ Matsuoka LY, Wortsman J, Haddad JG, Kolm P, Hollis BW (April 1991). "Racial pigmentation and the cutaneous synthesis of vitamin D". Arch Dermatol 127 (4): 536–8. doi:. PMID 1848745.
- ^ Sam D. Stout; Sabrina C. Agarwal; Stout, Samuel D. (2003). Bone loss and osteoporosis: an anthropological perspective. New York: Kluwer Academic/Plenum Publishers. ISBN 0-306-47767-X.
- ^ a b c Holick MF (01 Dec 2004). "Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease". American Journal of Clinical Nutrition Full Text 80 (6): 1678S–88S. PMID 15585788. http://www.ajcn.org/cgi/content/full/80/6/1678S.
- ^ a b Michael Holick, 5 May 2007, http://www.uvadvantage.org/portals/0/pres/
- ^ Nowson C, Margerison C (2002). "Vitamin D intake and vitamin D status of Australians". Med J Aust 177 (3): 149–52. PMID 12149085. http://www.mja.com.au/public/issues/177_03_050802/now10763_fm.html.
- ^ Grant WB, Holick MF (2005). "Benefits and requirements of vitamin D for optimal health: a review". Altern Med Rev 10 (2): 94–111. PMID 15989379.
- ^ Rajakumar K (2003). "Vitamin D, cod-liver oil, sunlight, and rickets: a historical perspective". Pediatrics 112 (2): e132–5. doi:. PMID 12897318. http://pediatrics.aappublications.org/cgi/content/full/112/2/e132.
- ^ Holick MF (February 2003). "Vitamin D: A millenium perspective". J. Cell. Biochem. 88 (2): 296–307. doi:. PMID 12520530.
- ^ Stewart B. Leavitt. "Vitamin D – A Neglected ‘Analgesic’ for Chronic Musculoskeletal Pain". Pain-Topics.org. http://pain-topics.org/pdf/vitamind-report.pdf. Retrieved on 2009-03-25.
- ^ Straube S, Andrew Moore R, Derry S, McQuay HJ (January 2009). "Vitamin D and chronic pain". Pain 141 (1-2): 10–3. doi:. PMID 19084336.
- ^ Natural Standard Research Collaboration (2008-03-01). "Vitamin D". Evidence-based monograph. Mayo Clinic. http://www.mayoclinic.com/health/vitamin-d/NS_patient-vitamind. Retrieved on 29 November 2008.
- ^ Gloth, F.M. 3rd; , Alam W, Hollis B. (1999). "Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder.". J Nutr Health Aging 3 (1): 5–7. pmid 10888476. http://www.ncbi.nlm.nih.gov/pubmed/10888476. Retrieved on 2008-11-15.
- ^ a b Melamed ML, Muntner P, Michos ED, et al (2008). "Serum 25-Hydroxyvitamin D Levels and the Prevalence of Peripheral Arterial Disease. Results from NHANES 2001 to 2004". Arterioscler. Thromb. Vasc. Biol. 28: 1179. doi:. PMID 18417640.
- ^ Llewellyn DJ, Langa K, Lang I (February 2009). "Serum 25-Hydroxyvitamin D Concentration and Cognitive Impairment". J Geriatr Psychiatry Neurol. doi:. PMID 19073839. http://jgp.sagepub.com/cgi/pmidlookup?view=long&pmid=19073839.
- ^ Evatt ML, Delong MR, Khazai N, Rosen A, Triche S, Tangpricha V (October 2008). "Prevalence of vitamin d insufficiency in patients with Parkinson disease and Alzheimer disease". Arch. Neurol. 65 (10): 1348–52. doi:. PMID 18852350. http://archneur.ama-assn.org/cgi/pmidlookup?view=long&pmid=18852350.
- ^ "RODENTICIDES, source: Journal of Veterinary Medicine, archives, vol. 27, May, 1998". IPM Of Alaska, Solving Pest Problems Sensibly. http://www.homestead.com/ipmofalaska/files/rodenticides.html. Retrieved on 2006-07-07.
- ^ Hathcock JN, Shao A, Vieth R, Heaney R (January 2007). "Risk assessment for vitamin D". Am. J. Clin. Nutr. 85 (1): 6–18. PMID 17209171. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=17209171. Free full=text.
- ^ Garrison RH, Somer E (1990) The Nutrition Desk Reference, Second Edition. p. 40. New Canaan, CT: Keats.
- ^ US Environmental Protection Agency. Cholecalciferol (Vitamin D3) Chemical Profile 12/84. Chemical Fact Sheet Number 42. Washington, DC. December 1, 1984.
- ^ a b c d Vieth R (01 May 1999). "Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety". Am J Clin Nutr 69 (5): 842–56. PMID 10232622. http://www.ajcn.org/cgi/content/full/69/5/842.
- ^ Bendich A, Langseth L (1989). J Clin Nutr Safety of vitamin A. 49. pp. 358–71. PMID 2492745. http://www.ajcn.org/cgi/content/full/83/2/191journal=Am J Clin Nutr.
- ^ 2004 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System.
- ^ "Complete Guide to Vitamins, Minerals ans Supplements", Fisher Books, Tucsan AZ, 1988, p42
- ^ Adams JS, Lee G (01 Aug 1997). "Gains in bone mineral density with resolution of vitamin D intoxication". Ann Intern Med 127 (3): 203–206. PMID 9245225. http://www.annals.org/cgi/content/full/127/3/203.
- ^ a b Rajasree S, Rajpal K, Kartha CC, Sarma PS, Kutty VR, Iyer CS, Girija G (2001). "Serum 25-hydroxyvitamin D3 levels are elevated in South Indian patients with ischemic heart disease Full Text". Eur J Epidemiol 17 (6): 567–71. doi:. PMID 11949730.
- ^ Holick M (1995). "Environmental factors that influence the cutaneous production of vitamin D". Am J Clin Nutr 61 (3 Suppl): 638S–645S. PMID 7879731.
- ^ a b Nagpal S, Na S, Rathnachalam R (August 2005). "Noncalcemic actions of vitamin D receptor ligands". Endocr. Rev. 26 (5): 662–87. doi:. PMID 15798098.
- ^ Janet Raloff, The Antibiotic Vitamin Science News, Vol 170, November 11, 2006, pages 312-317
- ^ Cannell JJ, Vieth R, Umhau JC, et al (2006). "Epidemic influenza and vitamin D". Epidemiol. Infect. 134 (6): 1129–40. doi:. PMID 16959053.
- ^ Nnoaham KE, Clarke A (2008). "Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis". Int J Epidemiol 37 (1): 113–9. doi:. PMID 18245055.
- ^ Gibney KB, MacGregor L, Leder K, et al (2008). "Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa". Clin. Infect. Dis. 46 (3): 443–6. doi:. PMID 18173355.
- ^ Martineau AR, Wilkinson RJ, Wilkinson KA, et al (2007). "A single dose of vitamin D enhances immunity to mycobacteria". Am. J. Respir. Crit. Care Med. 176 (2): 208–13. doi:. PMID 17463418.
- ^ Muhe L, Lulseged S, Mason KE, Simoes EA (June 1997). "Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children". Lancet 349 (9068): 1801–4. doi:. PMID 9269215.
- ^ Yee YK, Chintalacharuvu SR, Lu J, Nagpal S. (2005). "Vitamin D receptor modulators for inflammation and cancer". Mini Rev Med Chem. 5 (8): 761–78. doi:. PMID 16101412.
- ^ van Etten E, Mathieu C. (2005). "Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts". J Steroid Biochem Mol Biol. 97 (1-2): 93–101. doi:. PMID 16046118.
- ^ Munger KL. , Levin, LI,Hollis BW , Howard, NS , Ascherio A (2006). "Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis". Journal of the American Medical Association 296 (23): 2832–2838. doi:. PMID 17179460.
- ^ "Science News / Molecular Link Between Vitamin D Deficiency And MS". http://www.sciencenews.org/view/generic/id/40626/title/Molecular_link_between_vitamin__D_deficiency_and_MS. Retrieved on 2009-02-25.
- ^ Ingraham BA, Bragdon B, Nohe A (2007). "Molecular basis of the potential of vitamin D to prevent cancer". Curr Med Res Opin 24: 139. doi:. PMID 18034918.
- ^ "Vitamin D 'can lower cancer risk'". BBC News. 28 December 2005. http://news.bbc.co.uk/2/hi/health/4563336.stm. Retrieved on 2006-03-23.
- ^ Gorham ED, Garland CF, Garland FC et al. (2007). "Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 32:210-216.
- ^ Garland CF, Mohr SB, Gorham ED et al. (2006). "Role of ultraviolet B irradiance and vitamin D in prevention of ovarian cancer." Am J Prev Med. 31:512-514.
- ^ Freedman DM, Looker AC, Chang SC, Graubard BI (2007). "Prospective study of serum vitamin D and cancer mortality in the United States". J. Natl. Cancer Inst. 99 (21): 1594–602. doi:. PMID 17971526.
- ^ Tuohimaa P, Pukkala E, Scélo G, et al (2007). "Does solar exposure, as indicated by the non-melanoma skin cancers, protect from solid cancers: vitamin D as a possible explanation". Eur. J. Cancer 43 (11): 1701–12. doi:. PMID 17540555.
- ^ "Vitamin D 'aids lung cancer ops'". BBC News. 22 April 2005. http://news.bbc.co.uk/2/hi/health/4458085.stm. Retrieved on 2006-03-23.
- ^ Skinner HG, Michaud DS, Giovannucci E, Willett WC, Colditz GA, Fuchs CS (2006). "Vitamin D intake and the risk for pancreatic cancer in two cohort studies". Cancer Epidemiol. Biomarkers Prev. 15 (9): 1688–95. doi:. PMID 16985031. http://cebp.aacrjournals.org/cgi/content/full/15/9/1688.
- ^ BBC NEWS | Health | Vitamin D 'slashes cancer risk'
- ^ Martin Mittelstaedt (28 April 2007). "Vitamin D casts cancer prevention in new light". Global and Mail. http://www.theglobeandmail.com/servlet/story/RTGAM.20070428.wxvitamin28/BNStory/specialScienceandHealth/home. Retrieved on 2007-04-28.
- ^ Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. (2007). "Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial". Am J Clin Nutr. 85 (6): 1586–91. PMID 17556697.
- ^ Beer T, Myrthue A (2006). "Calcitriol in the treatment of prostate cancer". Anticancer Res 26 (4A): 2647–51. PMID 16886675.
- ^ a b Buyru N, Tezol A;,Yosunkaya-Fenerci E, Dalay, N. Vitamin D receptor gene polymorphisms in breast cancer. Experimental and Molecular Medicine. 2003; 35(6):550-555.
- ^ Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM. Intakes of calcium and vitamin D and breast cancer risk in women. Archives of Internal Medicine.2007; 167(10):1050-9.
- ^ Chen WY, Bertone-Johnson ER, Hunter DJ, Willett WC, Hankinson SE. Associations Between Polymorphisms in the Vitamin D Receptor and Breast Cancer Risk. Cancer Epidemiology, Biomarkers, & Prevention. 2005; 14(10):2335-2339.
- ^ Lack of vitamin D may increase heart disease risk http://www.americanheart.org/presenter.jhtml?identifier=3052800 American Heart Association rapid access journal report 01/07/2008
- ^ Newswise: Men with Low Vitamin D May Have Increased Risk of Heart Attack Retrieved on June 9, 2008.
- ^ Wang TJ, Pencina MJ, Booth SL, et al (January 2008). "Vitamin D deficiency and risk of cardiovascular disease". Circulation 117 (4): 503–11. doi:. PMID 18180395.
- ^ Lind L, Hänni A, Lithell H, Hvarfner A, Sörensen OH, Ljunghall S (September 1995). "Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men". Am. J. Hypertens. 8 (9): 894–901. doi:. PMID 8541004.
- ^ Grimes DS, Hindle E, Dyer T. (1996). "Sunlight cholesterol and coronary heart disease". Quarterly Journal of Medicine 89 (8): 579–589. PMID 8935479.
- ^ Spencer FA, Goldberg RJ, Becker RC, Gore JM. (1998). "Seasonal distribution of acute myocardial infarction in the second National Registry of Myocardial Infarction". J Am Coll Cardiol. 31 (6): 1226–33. doi:. PMID 9581712.
- ^ Ku CS, Yang CY, Lee WJ, Chiang HT, Liu CP, Lin SL. (1998). "Absence of a seasonal variation in myocardial infarction onset in a region without temperature extremes". Cardiology. 89 (4): 277–82. doi:. PMID 9643275.
- ^ Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E (1995). "Serum 25-hydroxyvitamin D3 is related to physical activity and ethnicity but not obesity in a multicultural workforce". Aust N Z J Med 25 (3): 218–23. PMID 7487689.
- ^ Melamed ML, Michos ED, Post W, Astor B (August 2008). "25-hydroxyvitamin D levels and the risk of mortality in the general population". Arch. Intern. Med. 168 (15): 1629–37. doi:. PMID 18695076.
- ^ Richards JB, Valdes AM, Gardner JP, et al (November 2007). "Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in women". Am. J. Clin. Nutr. 86 (5): 1420–5. PMID 17991655. PMC: 2196219. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=17991655.
[edit] Further reading
- Vitamin D Fact Sheet from the U.S.National Institutes of Health
- Vitamin D: An Old Vitamin With New Health Implications
- Vitamin D at the Open Directory Project
- History of Vitamin D
- The Vitamin D Council
- Tavera-Mendoza LE, White JH (November 2007). "Cell defenses and the sunshine vitamin". Sci. Am. 297 (5): 62–5, 68–70, 72. PMID 17990825. http://www.sciam.com/article.cfm?id=cell-defenses-and-the-sunshine-vitamin.
- CBC Radio, Quirks & Quarks, June 7, 2008, The Vitamin D Miracle Cure A Canadian science show hosted by Bob McDonald
- One hundred more scientific references
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||






